Abstract
This study was performed to evaluate the effects of conditioned media (CM) from human amniotic epithelial cells (HAECs) on the corneal wound healing process. Eighteen rabbits (36 eyes) were used and randomly assigned to three groups according treatment: CM from HAECs (group 1), vehicle alone (group 2), and saline (group 3). Corneal alkali injuries were induced with 1 N sodium hydroxide. Each reagent used for treatment evaluation was injected into the dorsal bulbar subconjunctiva and the area of the corneal epithelial defect was measured every other day. Two animals from each group were euthanized at a time on days 3, 7, and 15, and the cornea was removed for histological examination. The sum of the epithelial defect areas measured on day 0 to day 6 as well as day 0 to day 14 in group 1 was significantly smaller than those of other groups. Histological examination revealed that the group 1 corneas had less inflammatory cell infiltration and showed more intact epithelial features compared to the other groups. These results suggest that CM from HAECs promote corneal wound healing in rabbits.
It has been suggested that the transplantation of preserved human amniotic membrane (HAM) in severely damaged corneas might enhance ocular surface reconstruction [14]. HAM has been used to treat a wide variety of ocular surface disorders such as chemical or thermal burns, limbal stem cell deficiencies, bullous keratopathy, persistent corneal epithelial defects and perforation, pterygium, and symblepharon [20]. Human amniotic fluid (HAF) is contained in the amniotic sac lined by HAM. Most proteins in HAM have also been found in HAF, some of which are thought to originate from the HAM itself [23]. Therefore, HAF is also expected to be a useful tool for treating some ophthalmic conditions [1,11].
The exact action mechanism underlying the ability of both HAM and HAF to facilitate the healing and repair of corneal injuries has not been identified. However, it is believed that a number of cytokines and growth factors in both the epithelium and stroma of HAM affect the corneal healing process. Additionally, HAM may act as a substrate or scaffold for host cell migration and proliferation [16,20]. It has also been suggested that the positive effects of HAF on corneal wound healing are dependent on modulation by various growth factors and cytokines originating from HAM [11]. However, proteins in HAF are mainly ones in serum believed to be of maternal origin [12], and less than 5% of the total protein content in HAF is likely to have originated from HAM [6]. Therefore, there is possible that the positive effect of HAF on corneal wound healing might be due to substances from human serum.
Human serum contains several factors such as epidermal growth factor, vitamin A, transforming growth factor-β, fibronectin, and cytokines. These proteins are also found in tears and are important for maintaining a healthy corneal epithelium [8]. Therefore, it is necessary to identify amnion-specific factors and determine whether they have clinically positive effects on the corneal wound healing process. The purpose of this study was to evaluate the effects of conditioned medium (CM) derived from human amniotic epithelial cells (HAECs) on alkali-induced injuries in rabbit corneas.
Eighteen male New Zealand white rabbits were used in the study. All animals were treated according to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research (Animals in Research Committee, USA). The experimental protocol was approved by the Institute of Laboratory Animal Resources of Seoul National University, Korea (SNU-091015-2).
The rabbits were randomly assigned to three different groups according to the treatment protocol: CM from HAECs (group 1; n = 12), vehicle alone (group 2; n = 12), and saline (group 3; n = 12). Each reagent was injected in a volume of 0.2 mL into the dorsal bulbar subconjunctiva using a tuberculin syringe with 26-gauge needle on days 0 (4 h after the induction of corneal alkali injury), 1, and 2, and every other day thereafter until the animals were euthanized. A drop of 0.5% levoflaoxacin eye drops (Cravit; Santen, Japan) was administered to each eye after injection. Two animals in each group were euthanized on days 3, 7, and 15 by intracardiac injection of 10 mL potassium chloride (40 mEq/L) while under general anesthesia. The eyes were enucleated, and the corneas including a 2-mm scleral limb were excised and fixed in 10% buffered formalin for histological examination.
Human amniotic tissue was obtained from a human obstetric clinic (Korea University Guro Hospital, Korea) after acquiring informed consent. Isolation and culturing were performed with the approval of the Seoul National University Institutional Review Board (IRB No. 0611/001-002). The amniotic tissue was washed several times with phosphate buffered saline (PBS) (Cellgro; Corning, USA) to remove the blood, and incubated with 0.05% trypsin-EDTA (Invitrogen, USA) for 1 h. The HAECs were collected and suspended in standard culture media consisting of keratocyte serum-free medium (K-SFM) supplemented with 0.031 µg/µL recombinant human epidermal growth factor, 12.4 mg/mL bovine pituitary extract (all from Invitrogen, USA), and 10% fetal bovine serum (FBS; Thermo Fisher Scientific, USA). The HAECs were cultured until fully confluent in the standard culture media. The cells were then washed three times with PBS and further cultured with K-SFM without FBS or growth factors for 48 h. The media were collected, filtered through a 0.20-µm syringe filter (Minisart Plus; Sartorius, Germany), and stored at 4℃ before being used as CM. K-SFM without FBS or growth factors that had not been exposed to the HAECs was used as a vehicle control.
The rabbits were anesthetized with an intramuscular injection a tramadol-zolazepam-tiletamine-medetomidine solution (5.3 mg/kg, 3.3 mg/kg, 3.3 mg/kg, and 0.03 mg/kg, respectively). During the experimental procedure, the eyelids were held open with a wire speculum. After administration of a topical 0.5% proparacaine hydrochloride solution (Alcaine; Alcon, USA), a corneal vacuum trephine (Barron Radial Vacuum Trephine; Katena Products, USA) with an 8.5-mm diameter was attached to the cornea according to the manufacturer's instructions. After confirming that a vacuum was obtained, 0.5 mL of 1 N sodium hydroxide (NaOH) was poured into the trephine and left for 20 sec. The trephine was then irrigated thoroughly with a copious volume of lactated Ringer's solution (LRS) and the liquid in the trephine was simultaneously aspirated using a 5 French feeding tube connected to a suction line. After removing the trephine by releasing the vacuum, the eye was once again gently irrigated with LRS.
To monitor the corneal epithelial defects, fluorescein staining was performed immediately after inducing the alkali injury and every other day from days 0 to 14. A drop of 0.05% fluorescein was applied to stain the area of the epithelial defect, and excess dye was rinsed away with LRS. The eye was then photographed in dim light using a blue filtered digital camera (Canon Powershot G7; Canon, Japan). The area of the corneal epithelial defect was identified by bright green staining and measured using an image processing and analysis program (ImageTool; The University of Texas Health Science Center, USA).
Following fixation with 10% buffered formalin, corneas with 2-mm sclera limbs were embedded in paraffin and stained with hematoxylin and eosin according to standard protocols. The specimens were examined with an inverted microscope (10× magnification). Integrity of the corneal epithelium, infiltration of inflammatory cells, and the presence of blood vessels were evaluated.
A one-way analysis of variance (ANOVA) was used to compare the initial healing rates and epithelial defect areas measured in the different groups. Post-hoc analysis was performed using the least significant difference multiple comparisons test. P-values less than 0.05 were considered to be statistically significant. All statistical analyses were conducted with PASW Statistics software (ver. 17.0; IBM, USA).
On day 2, significant decreases in the epithelial defect area were observed in all groups (Fig. 1). The mean healing rate of each group during this period is presented in Table 1. Although the corneal epithelial healing rates for group 1 tended to be faster than those for the other groups, these differences were not statistically significant (p = 0.273). After the initial healing period, the corneal epithelial defect area increased in all groups on day 4. At this time, the corneal defect area of group 1 decreased greatly on day 6 and remained low up to day 12. Although the corneal defect area for group 2 decreased slowly until day 8, it increased again on day 10 and remained the highest of all groups up to day 12. The defect area of group 3 was the medium value among the groups from days 6 to 12 (Figs. 1 and 2).
To compare the effects of each treatment on corneal epithelial healing, the epithelial defect areas measured on day 0 and each time point were summed and the mean values were compared (Table 2). Differences in the mean sum of epithelial defect areas measured from day 0 to day 2 were not statistically significant between the groups (p = 0.304). However, the sum of epithelial defect areas for group 1 was significantly smaller than those of the other groups measured on day 6 (p = 0.003) and day 14 after injury (p = 0.027).
Table 3 and Fig. 3 show the corneal histological examination results. All corneas in groups 1 and 2 had intact epithelium on day 3 whereas group 3 had only two corneas with intact epithelium. In group 3, one cornea had epithelial features indicative of dysmaturation and poor attachment to the anterior stroma. On day 7, three corneas in group 1 had intact epithelium whereas other groups had two intact corneas. One cornea in group 2 showed epithelial blister formation while one cornea in group 3 had epithelial dysmaturation and non-adherence. Inflammatory cells infiltrated the stroma of the central cornea in groups 2 and 3. In contrast, infiltration of inflammatory cells in group 1 occurred only in the stroma of the peripheral cornea. On day 15, groups 1 and 2 had three corneas each with intact epithelium while all corneas in group 3 were de-epithelialized. The epithelium of one cornea in group 3 showed features of dysmaturation and non-adherence.
In the present study, we induced corneal injuries with 1 N NaOH because alkali burns affect all corneal layers and elicit more potent inflammatory reactions than other types of corneal injuries such as mechanical abrasions or trauma. Additionally, injuries were induced in the present study with corneal vacuum trephines rather than disks of filter paper as previously used by many researchers [3,4,15,17,21,22]. The reason for this was because the lesions inflicted with the trephines had clearer margins and precisely rounded areas than ones induced by the disks. Because the initial wound area and shape of the epithelial defect could affect the healing rate of the cornea [18], it might be possible for the epithelial healing process to be assessed more precisely by using corneal vacuum trephines.
In our study, reagents used for treatment were injected subconjunctivally because we expected the drug levels to be high and sustained in the cornea and anterior eye segment. Delivery of the treatments from the subconjunctival injection site to the anterior segment of the eye may have been achieved through direct diffusion as well as leakage back out the injection tract [5]. Therefore, the subconjunctival route enabled the delivery of treatments with longer duration of action and more advanced permeability into the anterior segment, including the cornea and limbus, compared to topical application.
Although the epithelium of alkali-injured corneas heals and covers the wound within 48 h, it always breaks down after the initial healing process [3]. These recurrent epithelial defects tend to consistently increase in size and persist indefinitely [19]. The corneas of all groups in the present study significantly re-epithelialized during the first 48 h, but de-epithelialized again over the next 48 h. For this reason, we calculated the initial healing rates for all corneas during the first 48 h. No statistically significant difference in these healing rates was observed between the groups.
Because corneal epithelial cells seemed to have sufficient regeneration and migration capacities for re-epithelialization even without therapeutic intervention [2,3], comparison of the initial healing rate is not very meaningful. Instead, it is more informative to evaluate the degree of recurrent epithelial injury after the initial healing period because the epithelium always subsequently breaks down in alkali-injured corneas [3]. We therefore calculated the sum of epithelial defect areas measured on day 0 and at each time point, and compared the values between groups. Using this method, the sum of epithelial defect areas measured at the respective time point might significantly increase to a greater extent if the epithelial defect recurred after the initial healing process. During the first 2 days of the initial healing period, there was no significant difference in the sum of the epithelial defect areas between the groups. However, the sum of epithelial defect areas for group 1 was significantly smaller than those of the other groups from days 0 to 6 and days 0 to 14. This means that CM from the HAECs inhibited the recurrence of epithelial wounds although the initial healing rates were similar in all groups.
Histological findings in the present study also demonstrated the positive effects of CM on corneal epithelial healing. At each time point, group 1 had more or the same number of corneas with intact epithelium compared to the other groups. In group 2, epithelial blister formation was observed in two corneas on day 7. Corneal epithelial features indicative of dysmaturation and detachment from the anterior stroma were also identified in three corneas from group 3. These epithelial features observed in group 3 may have accounted for the insignificant differences in the mean sum of epithelial defect areas between groups 1 and 3. Given that the non-adherent corneal epithelia in group 3 prevented the fluorescein dye from staining the anterior stroma, it might be possible that the epithelial defect areas of this group were under-estimated. Furthermore, infiltration of inflammatory cells into the corneal stroma was obviously reduced on day 7 in group 1. Less corneal neovascularization was also observed in group 1 compared to the other groups. These findings were evidence of the anti-inflammatory effects of the CM from HAECs on alkali-injured corneas.
Based on the findings of our study, it is more likely that the CM from HAECs influence epithelial-stromal interactions rather than initial epithelial healing, and inhibits the inflammatory process in alkali-injured corneas. These effects are believed to depend upon various soluble factors derived from HAECs. Indeed, it has been suggested that epidermal growth factor, keratinocyte growth factor, hepatocyte growth factor, and basic fibroblast growth factor originate from the amniotic epithelium [16]. In addition, it has been reported that amniotic epithelium expresses interleukin-1 receptor antagonist (IL-1ra), interleukin-10, and tissue inhibitors of metalloproteinase [10]. Furthermore, CM from HAECs contains proteins other than IL-1ra that suppress corneal inflammation [13]. Most of these factors are potentially involved in epithelial-stromal interactions in the ocular surface, and therefore play a crucial role in the process of corneal wound healing such as epithelial wound closure and stromal thinning [7,9].
In summary, our findings demonstrated that CM from HAECs exerts positive effects on wound healing in a model of alkali corneal injury. These effects include delaying epithelial breakdown after the initial healing procedure and anti-inflammatory activity.
Figures and Tables
Fig. 1
Changes in the mean ± SD corneal epithelial defect area after alkali injury in rabbits. Although the epithelial defect area decreased significantly on day 2, the area increased on day 4 in all groups. The area of group 1 (—) corneas then decreased and was maintained at a low value up to day 12. Although the area of group 2 (····) decreased slowly up to day 8, it increased again on day 10 and continued to be the highest among all groups up to day 12. The area of group 3 (----) had the medium value among the groups from days 6 to 12.

Fig. 2
Representative eyes from each group showing the epithelial defect area (bright green staining) and corneal neovascularization (white arrows on day 14). Note that the epithelial defect areas in all groups were greatly reduced within 2 days but enlarged again on day 4. Although the corneal epithelium in group 1 was intact from day 6 to 12, corneas in the other groups were recurrently de-epithelialized.
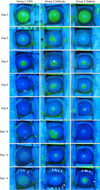
Fig. 3
Corneal histological changes observed on each day after alkali injury in the rabbits. On day 3, intact epithelium was seen in groups 1 (A) and 2 (B) while epithelial features of dysmaturation and poor attachment were found in group 3 (C). On day 7, intact epithelium was observed in group 1 (D), blister formation was seen on the alkali wound surface of a cornea in group 2 (E; white arrow), and epithelial dysmaturation and non-adherence of a cornea was found in group 3 (F; white arrow). On day 15, intact epithelium was seen in group 1 (G) and group 2 (H). Epithelial features of dysmaturation and de-epithelialization of a cornea in group 3 (I; white arrow). Black arrows indicate inflammatory cells infiltrated into the stroma in each group. H&E stain, ×10.
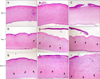
Table 2
Mean ± SD sum of corneal epithelial defect areas from day 0 to each time point after alkali injury in rabbits

*Data are expressed as the mean ± SD. †, ‡, §, ∥, ¶, **Values with different superscript symbols (i.e., † vs. ‡, § vs. ∥ and ¶ vs. **) are significantly different (p < 0.05) based on the least significant difference multiple comparisons test. ††Statistical significance was tested with a one-way ANOVA.
Acknowledgments
This study was supported through the BK21 Program for Veterinary Science, College of Veterinary Medicine, Seoul National University, Korea.
References
1. Castro-Combs J, Noguera G, Cano M, Yew M, Gehlbach PL, Palmer J, Behrens A. Corneal wound healing is modulated by topical application of amniotic fluid in an ex vivo organ culture model. Exp Eye Res. 2008. 87:56–63.

2. Chang CY, Green CR, McGhee CNJ, Sherwin T. Acute wound healing in the human central corneal epithelium appears to be independent of limbal stem cell influence. Invest Ophthalmol Vis Sci. 2008. 49:5279–5286.

3. Chung JH, Fagerholm P, Lindström B. The behaviour of corneal epithelium following a standardized alkali wound. Acta Ophthalmol (Copenh). 1987. 65:529–537.

4. Chung JH, Park YK, Paek SM, Chong YH, Kim WK. Effect of Na-hyaluronan on stromal and endothelial healing in experimental corneal alkali wounds. Ophthalmic Res. 1999. 31:432–439.

5. Conrad JM, Robinson JR. Mechanisms of anterior segment absorption of pilocarpine following subconjunctival injection in albino rabbits. J Pharm Sci. 1980. 69:875–884.

6. Drøhse H, Christensen H, Myrhøj V, Sørensen S. Characterisation of non-maternal serum proteins in amniotic fluid at weeks 16 to 18 of gestation. Clin Chim Acta. 1998. 276:109–120.

7. Gabison EE, Huet E, Baudouin C, Menashi S. Direct epithelial-stromal interaction in corneal wound healing: role of EMMPRIN/CD147 in MMPs induction and beyond. Prog Retin Eye Res. 2009. 28:19–33.

8. Geerling G, Maclennan S, Hartwig D. Autologous serum eye drops for ocular surface disorders. Br J Ophthalmol. 2004. 88:1467–1474.

9. Grueterich M, Espana EM, Tseng SCG. Ex vivo expansion of limbal epithelial stem cells: amniotic membrane serving as a stem cell niche. Surv Ophthalmol. 2003. 48:631–646.

10. Hao Y, Ma DH, Hwang DG, Kim WS, Zhang F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea. 2000. 19:348–352.

11. Herretes S, Suwan-Apichon O, Pirouzmanesh A, Reyes JMG, Broman AT, Cano M, Gehlbach PL, Gurewitsch ED, Duh EJ, Behrens A. Use of topical human amniotic fluid in the treatment of acute ocular alkali injuries in mice. Am J Ophthalmol. 2006. 142:271–278.

12. Johnson AM, Umansky I, Alper CA, Everett C, Greenspan G. Amniotic fluid proteins: maternal and fetal contributions. J Pediatr. 1974. 84:588–593.

13. Kamiya K, Wang M, Uchida S, Amano S, Oshika T, Sakuragawa N, Hori J. Topical application of culture supernatant from human amniotic epithelial cells suppresses inflammatory reactions in cornea. Exp Eye Res. 2005. 80:671–679.

14. Kim JC, Tseng SC. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995. 14:473–484.

15. Kim JS, Kim JC, Na BK, Jeong JM, Song CY. Amniotic membrane patching promotes healing and inhibits proteinase activity on wound healing following acute corneal alkali burn. Exp Eye Res. 2000. 70:329–337.

16. Koizumi N, Inatomi T, Sotozono C, Fullwood NJ, Quantock AJ, Kinoshita S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000. 20:173–177.

17. Kuznetsov SL, Nikolaeva LR, Spivak IA, Chentsova EV, Poltavtseva RA, Marei MV, Sukhikh GT. Effect of transplantation of cultured human neural stem and progenitor cells on regeneration of the cornea after chemical burn. Bull Exp Biol Med. 2006. 141:129–132.

18. Matsuda M, Ubels JL, Edelhauser HF. A larger corneal epithelial wound closes at a faster rate. Invest Ophthalmol Vis Sci. 1985. 26:897–900.
19. Pfister RR, Burstein N. The alkali burned cornea I. Epithelial and stromal repair. Exp Eye Res. 1976. 23:519–535.

20. Rahman I, Said DG, Maharajan VS, Dua HS. Amniotic membrane in ophthalmology: indications and limitations. Eye (Lond). 2009. 23:1954–1961.

21. Shahriari HA, Tokhmehchi F, Reza M, Hashemi NF. Comparison of the effect of amniotic membrane suspension and autologous serum on alkaline corneal epithelial wound healing in the rabbit model. Cornea. 2008. 27:1148–1150.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download