Abstract
In sheep, susceptibility to scrapie is mainly determined by codons 136, 154, and 171 of the PRNP gene. Five haplotypes are usually present (ARR, ARQ, ARH, AHQ, and VRQ). The ARR haplotype confers the greatest resistance to classical scrapie while VRQ renders animals most susceptible. In 2004, the European Union implemented a breeding program that promotes selection of the ARR haplotype while reducing the incidence of VRQ. From 2006 to 2011 in Belgium, frequency for the ARR/ARR genotypes increased from 38.3% to 63.8% (n = 6,437), the ARQ haplotype diminished from 21.1% to 12.9%, and the VRQ haplotype decreased from 2.0% to 1.7%. The status of codon 141, a determinant for atypical scrapie, was also evaluated. Out of 27 different breeds (n = 5,163), nine were abundant. The ARR/ARR frequency increased in eight of these nine major breeds. The selection program has had a major impact on the ARR haplotype frequency in Belgium. However, the occurrence of atypical scrapie represents a critical point for this program that warrants the continuous monitoring of scrapie. Additionally, genotype frequencies among the breeds varied greatly. Texel, a breed that is common in Belgium, can still be selected for due to its average ARR frequency.
Transmissible spongiform encephalopathies (TSEs) are a group of fatal neurodegenerative diseases caused by the accumulation of host-encoded prion protein (PrP) with an abnormal conformation mainly in the nervous system [33]. TSEs occur naturally in small ruminants. One example of is scrapie in sheep, which is the oldest known TSE first reported in the 18th century [9,26]. Unlike bovine spongiform encephalopathy (BSE), scrapie is not directly transmitted to humans [39]. However, scrapie prions are extremely resistant to degradation and can remain infectious for years in the natural environment [17].
In sheep, susceptibility to scrapie is determined by host genetic factors [18]. More precisely, this susceptibility is mainly modulated by amino acid polymorphisms encoded by the PrP gene (PRNP) at codons 136, 154, and 171 [1,3,12,23,24,38]. These polymorphisms are as follows: alanine (A) or valine (V) at codon 136, arginine (R) or histidine (H) at codon 154; and glutamine (Q), R or H at codon 171. Haplotypes for these three codons are commonly noted by three single-letter amino acid codes in the order of the codons (e.g., VRQ).
In Belgium, only the following five haplotypes are commonly present (listed in decreasing frequency): ARR, ARQ, ARH, AHQ, and VRQ [34]. The 15 most common genotypes have been classified into five groups of decreasing resistance (Fig. 1). The ARR/ARR genotype confers the highest resistance to the classical form of scrapie followed by the ARR heterozygotes except for ARR/VRQ [12,23]. The VRQ haplotype is associated with the greatest susceptibility [2].
In 1998, an atypical form of scrapie was discovered in Norway and named Nor98 [5]. Since then, this type of scrapie has been described in several European Union (EU) member states including Belgium [6,7,15]. It is not yet clear whether Nor98 is a transmissible disease in the field or whether it represents a sporadic disease. Genetic resistance to this specific form is not the same as that observed for classical scrapie. Sheep with the ARR/ARR genotype do not seem to have resistance to Nor98. Cases of Nor98, including those occurring in Belgium, have been described in animals with ARR/ARR genotypes [8,28]. However, ARR/ARR sheep are not the most susceptible to atypical scrapie since a polymorphism situated at a fourth codon [leucine (L) or phenylalanine (F) at codon 141] also affects susceptibility to this form of scrapie [32]. Most atypical scrapie cases have been detected in sheep with F at codon 141 (F141). It seems that the haplotype ARQ with F141 (noted as AF141RQ) is associated with greater susceptibility to atypical scrapie [28,35]. Similarly, the AL141HQ haplotype confers susceptibility to atypical scrapie [31]. In order to eradicate classical scrapie, the EU has promoted a breeding program since 2004 to select for ARR haplotypes and eliminate VRQ haplotypes from sheep populations [13]. It is known that selecting for scrapie-resistant sheep does not seem to have adverse effects on the production or health of the animals [13,40]. Lambs with Q/R171 alleles grow even faster than Q/Q171 lambs [14]. However, except for in the Netherlands [20] few data are available on the effectiveness of ARR haplotype selection as a function of time. This information is essential for assessing the current effect of the program as a whole. For this reason, the present study was carried out to evaluate the impact of the EU breeding scheme on the occurrence of resistant PRNP genotypes in Belgian sheep from 2006 to 2011. The effectiveness of selecting for the ARR/ARR genotypes and the influence of sheep breed on genotype frequencies were evaluated.
Samples included in this study had been sent to the National Reference Laboratory for routine genotyping analysis. These were either non-coagulated blood samples sent by the owners of living sheep to determine the genotype for future breeding purposes, or blood and brain samples supplied by an institution involved in TSE surveillance (e.g., a diagnostic laboratory or slaughterhouse). This second type of sample was from sheep slaughtered for human consumption or that had died from natural causes.
Commercial kits were used to isolate genomic DNA from blood (UltraClean BloodSpin; MO BIO Labs, USA) or brain (DNeasy; Qiagen, Germany) samples. Genotyping of codons 136, 154, and 171 was carried out with real-time (RT)-PCR (iCycler; Bio-Rad, USA) consisting of an activation of Uracil DNA-glycosylase at 50℃ (for 2 min), a DNA denaturation at 95℃ (8 min 30 sec) and 40 denaturation cycles at 95℃ (15 sec) and 60℃ (1 min). The codon 141 genotype was determined for a subset of the samples using the same RT-PCR technique but that was developed later for this codon. For each codon, the DNA was amplified with the reverse and forward sequence detection primers mentioned in Table 1 (Applied Biosystems, USA). The 136 primers were also used for codon 141 genotyping. For codon interpretation, two TaqMan MGB probes (three for codon 171 with three possible amino acids) were used with Universal PCR Master Mix without AmpErase Uracil.DNA glycosylase (Life Technologies, USA; Table 1).
For the brain samples that are often of low quality, genotyping data were confirmed by restriction fragment length polymorphism (RFLP) and electrophoresis on a denaturing gradient gel (DGGE). For these samples, the fragment with the codons was first amplified by PCR with two primers (P8: CAG GTT AAC GAT GGT GAA AAG CCA CAT AGG and P143: CTG GGA TTC TCT CTG GTA CT, Life Technologies): 95℃ for 10 min, 41 cycles of 94℃ for 1 min, 58℃ for 1 min, 72℃ for 1 min and then 72℃ for 7 min. The amplicons were then digested with BspHI (37℃ for 2 h) and the fragments were resolved on an acrylamide gradient gel containing formamide and urea as denaturants. The genotypes were determined by comparison with known samples from older analyses with RT-PCR.
The data set consisted of findings from 6 complete years (from January 2006 to December 2011). Genotypes for a total of 6,437 sheep were determined. Gender was known for 5924 of the sheep and the birth year was available for 1765 sheep. The analysis of codon 141 was limited to sheep carrying at least one ARQ haplotype that were still available in 2010 when the RT-PCR was developed for this codon in our lab. Statistical analyses were carried out with MyStat 12 (Systat Software, USA). Frequency changes between the two halves of the study period were verified by one-tailed 2 × 2 Chi-square tests (p-value < 0.05).
In 2006, the ARR haplotype was found in 61.4% of the sheep population analyzed; this number increased to 79.3% in 2011 (Fig. 2). During the same time period, the ARQ haplotype frequency diminished from 21.1% to 12.9% (Fig. 2) along with the VRQ haplotype frequency (2.0% to 1.7%; one-tailed Chi-square, n = 12,874, χ2 = 227, p = 0.015). Likewise, the frequency of two rare homozygotic genotypes remained low (AHQ/AHQ: 0.23% to 0.29% and VRQ/VRQ: 0.08% to 0.10%). In brief, during these 6 years of selection the frequency of ARR/ARR sheep was multiplied by almost 1.7 (from 38.3% to 63.8%, one-tailed Chi-square test, n = 12,874, χ2 = 227, p < 0.000; Fig. 1). ARR/ARR was the only genotype to increase in frequency. Over the years, the proportion of males among the genotyped sheep fluctuated between 40.5% and 57.2% with an overall average of 48.7%.
Additionally, the sheep that were born more recently showed a higher ARR haplotype frequency (increasing from 35.1% to 83.1%; Fig. 3). Based on the birth year, the average age of the animals tested was similar throughout the 6 years of the study (average: 493 +/- 82 days). The amino acid encoded by codon 141 was identified for 238 sheep carrying the ARQ haplotype: 97.9% L/L (n = 233), 2.1% L/F (n = 5), and no F/F.
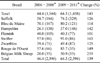
The breed was known for 5,163 (80.2%) of the sheep genotyped and 27 different breeds were analyzed. The majority of the animals belonged to nine breeds: Texel (the most common), Suffolk, Bleu du Maine, Hampshire, Flemish, Swifter, Zwartbles, Rouge de l'Ouest and Belgian Milk Sheep (Table 2). During the 6-year selection period, the number of ARR/ARR sheep increased for all the major breeds except among Flemish animals for which the ARR/ARR frequency remained about 40% (Table 3). These nine major breeds were roughly classified into three groups based on ARR/ARR genotype frequency and the frequency of genotype groups 3 or 5 (as defined in Fig. 1) as follows: first, breeds with the highest ARR/ARR frequencies but also the highest scrapie-sensitive ARR/VRQ frequencies (Swifter, Bleu du Maine, and to a lesser extent Rouge de l'Ouest; Table 4; second, breeds with average ARR/ARR frequencies (Suffolk, Texel, and Zwartbles); and finally breeds with the lowest ARR/ARR frequencies and highest ARQ/ARQ frequencies (Flemish, Belgian Milk Sheep, and Hampshire). The proportion of ARR haplotype significantly differed among these three groups (Chi-square test, n = 4,790, χ2 = 53.4, p < 0.000). The Belgian Milk Sheep breed was of further particular interest because these animals had a high proportion of ARR/AHQ.
Over the 6-year study period (2006 to 2011), the selection program for increasing resistance against classical scrapie had a major impact on the ARR haplotype frequency in the Belgian sheep population. This was particularly evidenced by the increasing probability of carrying the ARR haplotype among more recently born sheep. Moreover, the already very low VRQ allele frequency was diminished even further. It is not clear whether the increased proportion of ARR/ARR sheep in the Belgian population has limited the number of (classical) scrapie cases. In Belgium, 78 scrapie cases have been detected in sheep since 1992 and 46 have been found since 2002 [41]. The last positive case occurred in May 2007 (i.e., during the first third of our study period). This relatively low prevalence and an absence of cases after 2007 limited the analysis of the impact of the breeding program on scrapie occurrence in Belgium. However, it is clear that there has been a decline of scrapie frequency in Belgium similar to that in other European countries [19,20,37].
Even if classical scrapie prevalence has diminished since the beginning of the breeding scheme, a number of Nor98 cases have been detected. Out of the 78 Belgian scrapie cases originating from 25 primary cases, nine (11.5%) were atypical (unpublished data). The first atypical case was detected in Belgium in 2002 [8]. Eight of the nine last scrapie cases in Belgium were atypical (detected between 2004 and 2007, unpublished data). This concentration of atypical scrapie in recent years cannot be attributed to changes in the age cohort of the sheep tested since the average age remained the same. Two atypical cases occurred in ARR/ARR sheep while none were found in VRQ/- sheep (unpublished data). However, ARR/- sheep are not more susceptible to atypical scrapie than other sheep [4]. The most common haplotypes with atypical scrapie are AF141RQ, AL141HQ, and AL141RQ [32]. In the limited number of samples analyzed, the AF141RQ haplotype (susceptible to atypical scrapie) has been found only very rarely [32]. This could indicate good prospects for increasing resistance to atypical scrapie in the current Belgian sheep population. Indeed, no atypical scrapie has been detected in Belgium for about 5 years.
The identification of atypical scrapie cases is probably due to better surveillance techniques. Yet it could perhaps be linked to the selection of sheep with genotypes that confer resistance to classical but not atypical scrapie. This represents a critical point for the EU breeding program. The occurrence of atypical cases in the sheep population under selection justifies continued monitoring for scrapie prevalence. Since BSE can infect sheep, this monitoring is also essential for understanding the theoretical risk of BSE in the sheep population [16,21].
In Belgium, sheep with the ARR haplotype represented about 70% of the sheep selected in 2008 and 79% in 2011. In the Netherlands, the percentage of ARR haplotype sheep also increased during the selection program, and in 2008 these animals accounted for about 55% of the sheep under active surveillance [20]. Among Baltic breeds not under selection, the ARR/ARR genotype was found in between 9% and 31% of the sheep under active surveillance in the Baltic states [36].
Even though the main factor influencing scrapie susceptibility is the actual frequencies of the different susceptible haplotypes, sheep breed can also have an effect [29]. This was confirmed in the present study as the breeds greatly varied in genotype frequencies. For example, Swifter sheep showed the genetic profile most highly resistant to scrapie (75% were ARR/ARR). Other studies have demonstrated that even within a given breed, ARR/ARR frequency before selection is highly variable. For example, this frequency ranged from 19% to 58% in Suffolk [10,11,22,25,27]. In Belgium, the Suffolk sheep tested in our study ultimately developed an ARR/ARR genotype frequency of more than 74%. Texel sheep are very common in Belgium (58% of the samples in this study). This breed had an average proportion of highly resistant sheep (44% being ARR/ARR at the beginning of the study) and was the only breed in this study that included almost all genotypes. Texel sheep represent good opportunities for further selection since 92% of all these sheep in our study had the ARR haplotype. Similarly, Texel is also the most common breed in the Netherlands. About 56% of the farms contain Texel sheep (and possibly other breeds) followed by Swifter (30%~34%) and then Zwartbles (12%~16%) [30]. At the end of our study in Belgium, 64%, 93%, and 49% of these three breeds, respectively, were found to have the ARR/ARR genotype. To our knowledge, no genotyping data according to breed is available for the Netherlands, but on the whole 32% of the sheep on farms adhering to a selection program have the ARR/ARR genotype [20]. In Germany, 11% of Texel sheep are ARR/ARR before selection [10]. For undetermined reasons, the ARR/ARR frequency did not increase among Flemish sheep during the breeding program in Belgium. In conclusion, our results indicate that the Belgian sheep population is relatively scrapie-resistant although this can still be improved.
It is important to control goats along with sheep populations for a global approach to eradicate scrapie. More information on scrapie genetics in goats is required before the feasibility and advantages of such a breeding program can be assessed. This matter is currently being evaluated by the EU [42].
In summary, the breeding scheme for Belgian sheep has been very efficient in terms of increasing the proportion of ARR haplotypes within only a few years. It is not yet clear, however, whether this has led to decreased classical scrapie prevalence in Belgium. Nevertheless, it is apparent that in terms of prevalence atypical scrapie has almost replaced classical scrapie in recent years (2002~2007). Since 2007, no cases of scrapie, whether classical or atypical, have been observed in Belgium. However, the current sheep population is still amenable to further selection even if variations between the breeds result in differences in selection possibilities. In particular, the Texel, the major breed in Belgium, is still a good candidate for selection given that only 54% of Texel sheep have the ARR/ARR genotype as well as almost all other gnotypes.
Figures and Tables
Fig. 1
Changes in genotype frequencies during the breeding program in Belgium from 2006 to 2011. The genotypes are grouped into five categories of decreasing resistance to scrapie.

Fig. 2
Changes in the five haplotype frequencies during the breeding program in Belgium from 2006 to 2011.

Fig. 3
The influence of birth year on haplotype frequencies for the sheep analyzed. Due to their limited numbers, animals born before 2000 were grouped together. The number of sheep analyzed is indicated below each birth year.

Acknowledgments
The authors wish to thank Ms. Jessica De Sloovere for her valuable technical assistance. The authors also thank Ms. Riet Geeroms, Mr. Gaël Landuyt, Mr. Matthieu Pakula, Ms. Coralie Renard, and Mr. Patrick Van Muylem for making much of the study data available. The authors are thankful to three anonymous referees for their critical reading of the manuscript as well as Mr. Richard Sundahl for correcting the English language and grammar.
References
1. Baylis M, Chihota C, Stevenson E, Goldmann W, Smith A, Sivam K, Tongue S, Gravenor MB. Risk of scrapie in British sheep of different prion protein genotype. J Gen Virol. 2004. 85(Pt 9):2735–2740.

3. Belt PBGM, Muileman IH, Schreuder BEC, Bos-de Ruijter J, Gielkens ALJ, Smits MA. Identification of five allelic variants of the sheep PrP gene and their association with natural scrapie. J Gen Virol. 1995. 76(Pt 3):509–517.

4. Benestad SL, Arsac JN, Goldmann W, Nöremark M. Atypical/Nor98 scrapie: properties of the agent, genetics, and epidemiology. Vet Res. 2008. 39:19.

5. Benestad SL, Sarradin P, Thu B, Schönheit J, Tranulis MA, Bratberg B. Cases of scrapie with unusual features in Norway and designation of a new type, Nor98. Vet Rec. 2003. 153:202–208.

6. Buschmann A, Biacabe AG, Ziegler U, Bencsik A, Madec JY, Erhardt G, Lühken G, Baron T, Groschup MH. Atypical scrapie cases in Germany and France are identified by discrepant reaction patterns in BSE rapid tests. J Virol Methods. 2004. 117:27–36.

7. De Bosschere H, Roels S, Benestad SL, Vanopdenbosch E. Scrapie case similar to Nor98 diagnosed in Belgium via active surveillance. Vet Rec. 2004. 155:707–708.

8. De Bosschere H, Roels S, Dechamps P, Vanopdenbosch E. TSE detected in a Belgian ARR-homozygous sheep via active surveillance. Vet J. 2007. 173:449–451.

9. Dickinson AG, Stamp JT, Renwick CC. Maternal and lateral transmission of scrapie in sheep. J Comp Pathol. 1974. 84:19–25.

10. Drögemüller C, Leeb T, Distl O. PrP genotype frequencies in German breeding sheep and the potential to breed for resistance to scrapie. Vet Rec. 2001. 149:349–352.

11. Eglin RD, Warner R, Gubbins S, Sivam SK, Dawson M. Frequencies of PrP genotypes in 38 breeds of sheep sampled in the National Scrapie Plan for Great Britain. Vet Rec. 2005. 156:433–437.

12. Elsen JM, Amigues Y, Schelcher F, Ducrocq V, Andreoletti O, Eychenne F, Khang JV, Poivey JP, Lantier F, Laplanche JL. Genetic susceptibility and transmission factors in scrapie: detailed analysis of an epidemic in a closed flock of Romanov. Arch Virol. 1999. 144:431–445.

13. European Food Safety Authority. Opinion of the Scientific Panel on Biological Hazards on "the breeding programme for TSE resistance in sheep". EFSA J. 2006. 382:1–46.
14. Evoniuk JM, Berg PT, Johnson ML, Larson DM, Maddock TD, Stoltenow CL, Schauer CS, O'Rourke KI, Redmer DA. Associations between genotypes at codon 171 and 136 of the prion protein gene and production traits in market lambs. Am J Vet Res. 2007. 68:1073–1078.

15. Fediaevsky A, Tongue SC, Nöremark M, Calavas D, Ru G, Hopp P. A descriptive study of the prevalence of atypical and classical scrapie in sheep in 20 European countries. BMC Vet Res. 2008. 4:19.

16. Fryer HR, Baylis M, Sivam K, McLean AR. Quantifying the risk from ovine BSE and the impact of control strategies. Proc Biol Sci. 2007. 274:1497–1503.

17. Georgsson G, Sigurdarson S, Brown P. Infectious agent of sheep scrapie may persist in the environment for at least 16 years. J Gen Virol. 2006. 87(Pt 12):3737–3740.

18. Goldmann W. PrP genetics in ruminant transmissible spongiform encephalopathies. Vet Res. 2008. 39:30.
19. Gubbins S, McIntyre KM. Prevalence of sheep infected with classical scrapie in Great Britain, 1993-2007. Epidemiol Infect. 2009. 137:787–791.

20. Hagenaars TJ, Melchior MB, Bossers A, Davidse A, Engel B, van Zijderveld FG. Scrapie prevalence in sheep of susceptible genotype is declining in a population subject to breeding for resistance. BMC Vet Res. 2010. 6:25.

21. Häusermann C, Schwermer H, Oevermann A, Nentwig A, Zurbriggen A, Heim D, Seuberlich T. Surveillance and simulation of bovine spongiform encephalopathy and scrapie in small ruminants in Switzerland. BMC Vet Res. 2010. 6:20.

22. Hickford JGH, Zhou H, Fang Q, Byun SO, Gong H. Frequency of PRNP genotypes in common New Zealand sheep breeds. Vet Rec. 2008. 163:453–454.
23. Hunter N, Foster JD, Goldmann W, Stear MJ, Hope J, Bostock C. Natural scrapie in a closed flock of Cheviot sheep occurs only in specific PrP genotypes. Arch Virol. 1996. 141:809–824.

24. Hunter N, Goldmann W, Benson G, Foster JD, Hope J. Swaledale sheep affected by natural scrapie differ significantly in PrP genotype frequencies from healthy sheep and those selected for reduced incidence of scrapie. J Gen Virol. 1993. 74(Pt 6):1025–1031.

25. Ianella P, McManus CM, Caetano AR, Paiva SR. PRNP haplotype and genotype frequencies in Brazilian sheep: issues for conservation and breeding programs. Res Vet Sci. 2012. 93:219–225.

26. Jeffrey M, González L. Classical sheep transmissible spongiform encephalopathies: pathogenesis, pathological phenotypes and clinical disease. Neuropathol Appl Neurobiol. 2007. 33:373–394.

27. L'Homme Y, Leboeuf A, Cameron J. PrP genotype frequencies of Quebec sheep breeds determined by real-time PCR and molecular beacons. Can J Vet Res. 2008. 72:320–324.
28. Lühken G, Buschmann A, Brandt H, Eiden M, Groschup MH, Erhardt G. Epidemiological and genetical differences between classical and atypical scrapie cases. Vet Res. 2007. 38:65–80.

29. McIntyre KM, Trewby H, Gubbins S, Baylis M. The impact of sheep breed on the risk of classical scrapie. Epidemiol Infect. 2010. 138:384–392.

30. Melchior MB, Windig JJ, Hagenaars TJ, Bossers A, Davidse A, van Zijderveld FG. Eradication of scrapie with selective breeding: are we nearly there? BMC Vet Res. 2010. 6:24.

31. Moreno CR, Moazami-Goudarzi K, Laurent P, Cazeau G, Andreoletti O, Chadi S, Elsen JM, Calavas D. Which PrP haplotypes in a French sheep population are the most susceptible to atypical scrapie? Arch Virol. 2007. 152:1229–1232.

32. Moum T, Olsaker I, Hopp P, Moldal T, Valheim M, Moum T, Benestad SL. Polymorphisms at codons 141 and 154 in the ovine prion protein gene are associated with scrapie Nor98 cases. J Gen Virol. 2005. 86(Pt 1):231–235.

33. Prusiner SB. Molecular biology and pathogenesis of prion diseases. Trends Biochem Sci. 1996. 21:482–487.

34. Roels S, Renard C, De Bosschere H, Geeroms R, Van Poucke M, Peelman L, Vanopdenbosch E. Detection of polymorphisms in the prion protein gene in the Belgian sheep population: some preliminary data. Vet Q. 2004. 26:3–11.

35. Saunders GC, Cawthraw S, Mountjoy SJ, Hope J, Windl O. PrP genotypes of atypical scrapie cases in Great Britain. J Gen Virol. 2006. 87(Pt 11):3141–3149.

36. Sild E, Volskiene R, Viinalass H, Miceikiene I, Grislis Z, Distl O, Drögemüller C. Detection of prion protein gene polymorphisms in Baltic breeds of sheep. Vet Rec. 2006. 159:247–250.

38. Smits MA, Bossers A, Schreuder BEC. Prion protein and scrapie susceptibility. Vet Q. 1997. 19:101–105.

39. Spiropoulos J, Lockey R, Sallis RE, Terry LA, Thorne L, Holder TM, Beck KE, Simmons MM. Isolation of prion with BSE properties from farmed goat. Emerg Infect Dis. 2011. 17:2253–2261.

40. Sweeney T, Hanrahan JP. The evidence of associations between prion protein genotype and production, reproduction, and health traits in sheep. Vet Res. 2008. 39:28.

41. The European Commission. Cumulative TSE Testing in Sheep Since 2002. 2009. Brussels: The European Commission;1.
42. The European Commission. The TSE Road Map 2. 2010. Brussels: The European Commission;4–15.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download