Abstract
The purpose of this study was to determine whether osteoprotegerin (OPG) could affect osteoclat differentiation and activation under serum-free conditions. Both duck embryo bone marrow cells and RAW264.7 cells were incubated with macrophage colony stimulatory factor (M-CSF) and receptor activator for nuclear factor κB ligand (RANKL) in serum-free medium to promote osteoclastogenesis. During cultivation, 0, 10, 20, 50, and 100 ng/mL OPG were added to various groups of cells. Osteoclast differentiation and activation were monitored via tartrate-resistant acid phosphatase (TRAP) staining, filamentous-actin rings analysis, and a bone resorption assay. Furthermore, the expression osteoclast-related genes, such as TRAP and receptor activator for nuclear factor κB (RANK), that was influenced by OPG in RAW264.7 cells was examined using real-time polymerase chain reaction. In summary, findings from the present study suggested that M-CSF with RANKL can promote osteoclast differentiation and activation, and enhance the expression of TRAP and RANK mRNA in osteoclasts. In contrast, OPG inhibited these activities under serum-free conditions.
Bone is continuously and dynamically remodeled through two coupled processes: degradation of existing bone through the resorption activity of osteoclasts and deposition of new bone through the matrix formation activity of osteoblasts [30]. Regulation of the recruitment, proliferation, and differentiation of osteoclasts and osteoblasts is critical for the maintenance of bone mass [24]. Under many pathologic conditions including osteoporosis, osteosclerosis, and rheumatoid arthritis, the amount of bone removed and deposited by osteoblasts is unbalanced [37]. Osteoclasts, which are specialized monocyte/macrophage family members derived from bone marrow hematopoietic precursors [21], are the principal (if not exclusive) resorptive cells of bone [28]. RANKL and M-CSF are essential for the formation of osteoclasts [14].
To prevent pathological bone loss, factors that can inhibit the bone resorption activity of osteoclasts have been used as potential anti-resorptive agents. One example is OPG, which belongs to the tumor necrosis factor (TNF) receptor superfamily [32]. OPG is known to negatively regulate osteoclast maturation and activation [1]. This factor initially acts as a decoy receptor and prevents the interaction of RANKL with RANK, thereby curbing the activity, survival, and proliferation of osteoclasts [22]. OPG also directly inhibits osteoclast function through a RANKL/RANK/OPG axis-independent mechanism [17]. The inhibitory effects of OPG on osteoclast differentiation and activation have great significance in the prevention of bone loss diseases. To the best of our knowledge, the present study was the first to use a duck embryo bone marrow cell model to study osteoclasts. The present investigation was also in unique in that it involved the use of RAW264.7 cells to measure the expression of TRAP and RANK using a more accurate analytical real-time fluorescence quantitative polymerase chain reaction (PCR) method. Vincent et al. [35] reported a defined model for studying osteoclast differentiation and activity in the absence of serum. This model proved to be ideal for studying the role of agonistic and antagonistic molecules in the differentiation and activation of osteoclast. The induction of primary osteoclasts differentiation and activation (duck osteoclasts) under serum-free conditions has not been reported. The current study was performed to evaluate the OPG inhibitory effect on osteoclast differentiation and activation as well as the expression of osteoclast differentiation-related genes TRAP and RANK. We also wanted to lay the foundation for developing a potential OPG treatment protocol for preventing bone loss disease resulting from osteoclast dynamic bone resorption activity.
Normal 23-day-old Gaoyou duck embryos were kindly provided by Jiangsu Waterfowl Gene Pool of Quality Resources (Taizhou, Jiangsu province, China). The duck embryos were sacrificed by exsanguination (Jiangsu Province Posts Certificate of Experimental Animal Practitioners number 203118). Bone marrow cells were harvested as previously described with minor modifications [16]. The cells were adjusted at a concentration of 1 × 106 cells/mL and cultivated in minimum essential medium alpha (α-MEM; containing 10% fetal bovine serum [FBS]; Gibco BRL, USA) in a 37℃ incubator with 5% CO2 and saturated humidity. After 24 h, non-adherent cells were collected for TRAP-specific staining, filamentous (F)-actin-specific staining, and a pit formation assay. The cells were suspended in α-MEM (containing 10% FBS; Gibco BRL, USA) and then seeded in 96-, 12-, and 48-well culture plates (Corning, USA) with bovine cortical bone slices [The bovine cortical bone was sawed into slices by saw microtome (SP1600, Leica microsystems, Germany) in Shanghai Ninth People's Hospital Affiliated Shanghai JiaoTong University School of Medicine]. After a 24-h cultivation period, the previous α-MEM was replaced with serum-free α-MEM medium supplemented with human M-CSF (25 ng/mL; Peprotech, USA) and murine RANKL (30 ng/mL; Peprotech). The cells were then cultured in a 37℃ incubator with 5% CO2 and saturated humidity, for up to 5 days before 0, 10, 20, 50, and 100 ng/mL OPG (human OPG; Peprotech) were added in the presence of M-CSF and RANKL. The cells were incubated for an additional 5 days in a 37℃ incubator with 5% CO2 and saturated humidity.
Murine monocyte/macrophage RAW 264.7 cells were purchased from the American Type Culture Collection (Manassas, USA). The cells were cultured in Dulbecco's modified eagle medium (DMEM; containing 10% FBS; Gibco BRL, USA). For TRAP-specific staining, the cells were suspended in α-MEM containing 10% FBS and then seeded in 96-well culture plates at a concentration of 3 × 104 cells/mL. After 24 h of culturing, the previous α-MEM was replaced into serum-free α-MEM medium containing M-CSF (25 ng/mL) with RANKL (30 ng/mL). After 48 h, 0, 10, 20, 50, and 100 ng/mL OPG were added to various groups of cells in the presence of M-CSF and RANKL, and the cells were incubated for another 3 days.
Multinucleated cells were stained for TRAP, an enzyme highly expressed in mature osteoclasts and prefusion mononuclear precursors. TRAP (TRAP kit; Sigma-Aldrich, USA) staining was conducted according to the protocol. Multinucleated TRAP-positive cells containing at least three nuclei were identified as osteoclast-like cells using a light microscope (Leica Microsystems).
To visualize the actin cytoskeleton, tetramethyl rhodamine isothiocyanate (TRITC)-conjugated phalloidin (Invitrogen, USA) was used. At the end of the previous incubation, the cells that were protected from light were fixed, permeabilized, blocked, and stained with TRITC- conjugated phalloidin (20 µmol/L) for 20 min at 37℃. The stained cells were examined with an inverted phase contrast fluorescence microscope (Leica Microsystems) and the green filters.
Duck embryo bone marrow cells were seeded on 48-well plates with bovine cortical bone slices. After culturing, the remaining cells on the slices were lysed with 0.25 mol/L NH4OH. Excavations in the slice surface were observed with an environmental scanning electron microscope (XL30-ESEM; Philips, The Netherlands). Image analysis was performed to measure the pit resorption area (Version 1.0; JEDA Technologies, USA).
RAW264.7 cells were suspended in α-MEM containing 10% FBS and seeded in 6-well culture plates at a concentration of 3 × 104 cells/mL. After a 24-h cultivation period, the previous α-MEM was replaced with serum-free α-MEM medium with 25 ng/mL M-CSF and 30 ng/mL RANKL, and the cells were incubated for 48 h. For the concentration gradient study, 0, 10, 20, 50, and 100 ng/mL OPG were added in the presence of M-CSF and RANKL to various groups of cells. OPG treatment was sustained for 30 min; the control cells were treated with M-CSF and RANKL alone during this period. For the time gradient study, 100 ng/mL OPG was added in the presence of M-CSF with RANKL to the various groups of cells. OPG treatment was sustained for 15, 30, 60, and 120 min. Control cells were treated with M-CSF and RANKL alone for another 15 min.
Total RNA was extracted at the destined period as previously mentioned. Reverse transcription was performed with a PrimeScript RT reagent kit with gDNA Eraser (Takara Bio, Japan). Sequences of the PCR primers specific for TRAP, RANK, and GAPDH used for this assay are listed in Table 1. Real-time PCR was performed using a 7500 Real-Time PCR system (Applied Biosystems, USA). The PCR protocol for TRAP and RANK amplification consisted of 40 cycles of 95℃ for 30 sec and 57℃ for 1 min. All real-time PCR reactions were carried out at least three times, and specificity of the PCR products was verified with a melting curve analysis. The threshold cycle (Ct) value was calculated based on the amplification plots.
M-CSF and RANKL induced a high level of TRAP- positive multinucleated osteoclast differentiation in the duck embryo bone marrow cells and RAW264.7 cells in vitro (Figs. 1A~B4). OPG significantly inhibited osteoclast differentiation in a concentration-dependent manner. In the duck embryo bone marrow cells, 10, 20, 50, and 100 ng/mL OPG decreased the number of TRAP-positive multinucleated cells by 26.36 ± 10.25%, 28.40 ± 13.06%, 43.86 ± 14.65%, and 76.75 ± 9.52%, respectively (Fig. 1C). In RAW 264.7 cells, 10, 20, 50, and 100 ng/mL OPG reduced the number of TRAP-positive multinucleated cells by 19.93 ± 21.41%, 33.84 ± 21.44%, 54.85 ± 3.11%, and 81.87 ± 6.85%, respectively (Fig. 1D). Osteoclasts in both the duck embryo bone marrow and RAW264.7 cell populations had multiple nuclei (≥3). The nuclei were TRAP-negative with red TRAP-positive cytoplasm. However, the number of TRAP-positive multinucleated cells in the duck embryo bone marrow cell population was less than that observed among RAW264.7 cells. In addition, the average number of nuclei in the duck embryo bone marrow osteoclasts was less than that found in RAW264.7 cells.
OPG suppressed the formation of F-actin rings in the duck embryo osteoclasts (Fig. 2). In the control groups, the osteoclast cytoskeleton was regular with a clear boundary, thereby enriching the F-actin rings and ruffled borders. In contrast, the cytoskeletal structure profile was obscure and the F-actin ring was thin in the OPG-treated cells. Additionally, fewer F-actin rings were observed in the OPG-treated groups. With high concentrations of OPG, the fusion of bone marrow cells was incomplete and F-actin ring formation was inhibited.
Round, elliptical, botuliform, or irregular pit resorption patterns were observed in bone slices co-cultured with the duck embryo bone marrow cells. Slices that were cultured without cells were intact and smooth. OPG had a dose-dependent inhibitory effect on the osteoclast resorptive capacity (Fig. 3A~A5), compared to the control groups, 11.29 ± 8.06%, 40.73 ± 10.46%, 60.21 ± 24.51%, and 86.06 ± 3.50%, inhibition at 10, 20, 50, 100 ng/mL concentrations, respectively (Fig. 3A6).
Osteoclast-specific genes, such as RANK and TRAP, were highly expressed in differentiated RAW264.7 cells. This was confirmed by real-time fluorescence quantitative PCR. Results of the concentration gradient study showed that the expression of TRAP mRNA in the control groups was significantly higher than in cells cultured without M-CSF and RANKL (p < 0.01). However, the expression of RANK mRNA was unaffected. OPG decreased the expression of RANK and TRAP in M-CSF- and RANKL-treated RAW264.7 cells (Figs. 4A and B). At concentrations of 10, 20, 50, 100 ng/mL, OPG inhibited the expression of TRAP mRNA by 0.11 ± 0.08-, 0.17 ± 0.04-, 0.27 ± 0.05-, and 0.60 ± 0.06-fold, respectively, and reduced the expression of RANK mRNA by 0.21 ± 0.32-, 0.29 ± 0.27-, 0.34 ± 0.16-, and 0.48 ± 0.12-fold, respectively.
The time gradient study demonstrated that the expression of TRAP and RANK mRNA in the control groups was higher than in the cells cultured without any exogenous cytokines for an additional 15 min (0.75 ± 0.01 and 0.26 ± 0.13, respectively; Figs. 4C and D). After OPG was added, notable inhibition of TRAP and RANK mRNA expression (p < 0.01) was observed after 30 min. Compared to the control groups, expression of these factors was significantly lower in cells cultured with M-CSF and RANKL plus OPG for 30, 60, and 120 min (p < 0.05). After 30, 60, and 120 min of exposure, OPG inhibited the expression of TRAP mRNA by 0.93 ± 0.01-, 0.18 ± 0.08-, and 0.62 ± 0.04-fold, respectively. Interestingly, TRAP mRNA expression increased by 1.87 ± 0.40-fold after 15 min. OPG inhibited the expression of RANK mRNA by 0.05 ± 0.08-, 0.77 ± 0.04-, 0.89 ± 0.04-, and 0.87 ± 0.07-fold after 15, 30, 60, and 120 min of treatment, respectively.
A previous study [18] reported that serum proteins, such as α2HS-glycoprotein, may be important for osteoclasts differentiation and activity. To reduce the effects of unknown components in animal sera, the elimination or reduction of serum during the cell culture process is desirable. In the present study, the cells were therefore treated with M-CSF, RANKL, and OPG under serum-free conditions.
Bone marrow cells of human [12], mice [27], and chicken embryo [36] have been used to establish osteoclast models. At this time, no study on osteoclasts derived from duck bone marrow cells has been performed. Evidence has suggested that osteoclasts in poultry are more aggressive bone resorbers than their mammalian counterparts (e.g., rabbit and rat) [13]. Hence, poultry osteoclasts are superior models for osteoclast research.
Primary bone marrow cells and RAW264.7 cell line were used to investigate osteoclast differentiation and activation in the present study. The above idea was based on previous reports by Choi et al. [8] and Tang et al. [33]. To perform research under conditions that most closely mimic those found in vivo, a TRAP assay, F-actin rings analysis, and bone resorption study were conducted using duck embryo osteoclasts recovered from duck embryo bone marrow cells. It was difficult to obtain the isolated primary osteoclasts. Furthermore, the cells were not suitable for conducting more sensitive and accurate biochemical molecular studies. Fortunately, RAW264.7 cells have been extensively used as in vitro osteoclast precursors models [9]. These cells also retain the capacity to differentiate into osteoclast-like cells [7]. TRAP and RANK genes were expressed in RAW264.7 cells incubated with M-CSF and RANKL. Large quantities of pure cellular materials obtained from RAW264.7 cell cultures allowed us to conduct our investigation, which would have been impossible with purified primary osteoclasts.
TRAP-specific staining is widely used to identify osteoclasts in vivo and in vitro because of its simplicity and ease of manipulation [29]. Moreover, TRAP participates in osteoclast bone resorption and the TRAP expression increases when resorption is enhanced [31]. F-actin rings are specific cytoskeletal structures characteristic of polarized and active osteoclasts [15]. These rings are essential for bone resorption by osteoclasts [24]. F-actin rings observed in the duck embryo bone marrow cells were similar to those described in the study by Lee et al. [24]. The similar F-actin rings were found in human peripheral blood mononuclear cells (hPBMC), however, the F-actin rings in the duck embryo osteoclasts were larger than ones in hPBMC [21].
Bone resorption, which is a unique function of osteoclasts, is the most reliable feature for identifying osteoclasts and designing anti-osteoporosis therapeutic strategies that target this type of cell [34]. A previous study [11] was performed to examine mice osteoclasts and compared bone resorption activities of osteoclasts from duck embryos and mice. The data showed that osteoclasts from duck embryo posses more vigorous bone resorption abilities than those from mice. This result was in accordance with findings from a previous investigation [13].
In bone cells, the expression of RANK and TRAP can serve as a marker of the osteoclastic phenotype [19,25]. RANKL and M-CSF are expressed in osteoblasts/stromal cells, which is essential for osteoclastogenesis [21]. RANK is expressed in osteoclast precursors and stimulates osteoclast maturation [10]. In the present study, TRAP-positive multinucleated cells were observed among duck embryo bone marrow cells treated with M-CSF and RANKL. This result was similar to ones from a study by Hou et al. [20] who reported that RANKL and M-CSF induce OLC formation in chicken marrow cells. TRAP-positive cells were also observed among RAW264.7 cells incubated with M-CSF and RANKL. Moreover, osteoclast-related genes indicative of a mature phenotype such as TRAP and RANK were highly expressed during terminal differentiation in RAW264.7 cells. These results suggested that M-CSF and RANKL could promote osteoclast differentiation and activation. This finding is similar to one of a previous report by Cuetara et al. [9].
The human recombinant OPG used in the current study was also used for a duckling osteoclat study by Gu et al. [16]. Previous report [4] identified a high homology between the partial sequence of chicken OPG and human recombinant OPG. The present investigation demonstrated that OPG could inhibit osteoclast differentiation. This process was characterized by a reduction of TRAP-positive multinucleated cell numbers, the shrinking of F-actin rings, and decreased expression of TRAP and RANK. Moreover, OPG reduced the resorption activity of the osteoclasts. These results demonstrated that OPG had an inhibitory effect on osteoclast activation, consistent with the results from a study by Simonet et al. [32]. Itonaga et al. [21] reported that transforming growth factor β can induce osteoclast differentiation in RAW 264.7 cells via a mechanism independent of the RANKL/RANK/OPG axis, and OPG does not inhibit this process. This present study indicated that both duck embryo bone marrow cells and RAW 264.7 cells treated with M-CSF and RANKL differentiated into osteoclasts through the RANKL/RANK/OPG axis, and showed that OPG played an inhibitory role.
A previous report stated that OPG can regulate the expression of RANK mRNA in mouse osteoclast-like cells [6]. In the concentration and time gradient studies we conducted, OPG was found to attenuate RANK expression in RAW264.7 cells. This result was consistent with that of a study by Chen et al. [6]. However, the data in the present study showed that RANK mRNA expression levels with or without M-CSF and RANKL did not significantly differ. One interpretation of this result is that RANK genes are also highly expressed in osteoclast precursors [37]. In the time gradient study, OPG significantly increased the expression of TRAP mRNA after 15 min of treatment. The expression of TRAP, as a phenotypic marker of osteoclasts, is closely associated with the osteoclast state.
Osteoclasts take part in immune responses and secrete cytokines that can affect their own functions. Their role as immune cells or immune response modulators has more recently been clarified [2,3]. RAW264.7 cells became unresponsive to stimulation in the short term. These cells act as osteoclast precursors not by only responding to TNF but also secrete TNF and other cytokines such as interleukin-6 (IL-6) and interleukin-1 (IL-1) [2]. IL-1 is produced by RAW264.7 monocytes/macrophages in anti-stimulation action [26]. IL-1 promotes osteoclast differentiation in RAW264.7 cells incubated with M-CSF and RANKL [23]. Moroever, Chamoux et al. [5] reported that OPG can inhibit osteoclast apoptosis although this compound is known to inhibit cell differentiation and activation, and may promote osteoclast apoptosis [37]. Details about the influence of OPG on osteoclasts remain controversial and will require further investigation to clarify.
Taken together, findings from the present study suggested that M-CSF and RANKL promote osteoclast differentiation and activation. These compounds also enhanced the expression of TRAP and RANK mRNA in the osteoclasts. In contrast, OPG inhibited the expression of these factors under serum-free conditions. The results may lay the foundation for OPG in the treatment of osteopenic disorders in duck that are characterized by excessive osteoclast activity.
Figures and Tables
Fig. 1
Inhibitory effects of OPG on TRAP-positive multinucleated osteoclast differentiation in M-CSF- and RANKL-stimulated duck embryo bone marrow and RAW 264.7 cells. (A~A4) Duck embryo bone marrow cells were suspended in α-MEM with FBS (v/v 10%) and then seeded in 96-well culture plates. After a 24-h cultivation period, the α-MEM was replaced with serum-free medium containing M-CSF (25 ng/mL) and RANKL (30 ng/mL). The cells were cultured up to 5 days. Next, 0, 10, 20, 50, and 100 ng/mL OPG were added to various groups of cells in the presence of M-CSF and RANKL, and incubated for another 3 days. (B~B4) RAW 264.7 cells were suspended in α-MEM with FBS (v/v 10%) and then seeded in 96-well culture plates. After a 24-h culturing period, the α-MEM was replaced with serum-free medium with M-CSF (25 ng/mL) and RANKL (30 ng/mL). The cells were then incubated for 48 h. Next, 0, 10, 20, 50, and 100 ng/mL OPG were added to various groups of cells in the presence of M-CSF and RANKL, and the cells were incubated for another 3 days. At the end of the incubation period, the two types of cells were fixed and stained for TRAP. (A and B) control, (A1 and B1) 10 ng/mL OPG, (A2 and B2) 20 ng/mL OPG, (A3 and B3) 50 ng/mL OPG, (A4 and B4) 100 ng/mL OPG. ×50 (A~A4), ×100 (B~B4). Scale bars = 500 µm (A~A4), 200 µm (B~B4). (C and D) TRAP-positive multinucleated cells (TRAP+) were observed under a light microscope and counted. The results are expressed as the mean ± SEM. **p < 0.01 and *p < 0.05 vs. the control groups. OPG: osteoprotegerin, TRAP: tartrate-resistant acid phosphatase, M-CSF: macrophage colony stimulatory factor, RANKL: receptor activator for nuclear factor κB ligand, α-MEM: minimum essential medium alpha, FBS: fetal bovine serum.
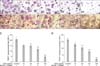
Fig. 2
Suppressive effects of OPG on F-actin ring formation in duck embryo osteoclasts. Duck embryo bone marrow cells were suspended in α-MEM with FBS (v/v 10%) and then seeded in 12-well culture plates. After incubating for 24 h, the α-MEM was replaced with serum-free medium containing M-CSF (25 ng/mL) and RANKL (30 ng/mL). The cells were cultured for up to 5 days. Next, 0, 10, 20, 50, and 100 ng/mL OPG were added to the different groups of cells in the presence of M-CSF and RANKL. The cells were incubated for another 3 days. At the end of the cultivation period, the cells were fixed and stained for TRITC-phalloidin. The F-actin rings were observed using an inverted phase contrast fluorescence microscope with appropriate filters. (A) control, (B) 10 ng/mL OPG, (C) 20 ng/mL OPG, (D) 50 ng/mL OPG, (E) 100 ng/mL OPG. ×400. Scale bars = 100 µm (A~E).
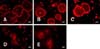
Fig. 3
Inhibition of osteoclasts pit resorption by OPG. (A~A5) Duck embryo bone marrow cells were suspended in α-MEM with FBS (v/v 10%) and then seeded in 48-well culture plates with bovine cortical bone slices. After 24 h, the α-MEM was replaced with serum-free medium supplemented with M-CSF (25 ng/mL) and RANKL (30 ng/mL). The cells were cultured for up to 5 days. Next, 0, 10, 20, 50, 100 ng/mL OPG were added to the different groups of cells in the presence of M-CSF and RANKL, and incubated for another 3 days. At the end of the incubation period, resorbed areas were examined using an environmental scanning electron microscope. The groups were as follows: (A) control, (A1) 10 ng/mL OPG, (A2) 20 ng/mL OPG, (A3) 50 ng/mL OPG, (A4) 100 ng/mL OPG, and (A5) bone slices without cells. ×1,000. Scale bars = 50 µm. (A6) The histogram represents the volume of pit resorption. The results are expressed as the mean ± SEM. **p < 0.01 and *p < 0.05 vs. the control groups.
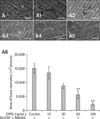
Fig. 4
Effects of OPG on the expression of TRAP and RANK. RAW264.7 cells were suspended in α-MEM with FBS (v/v 10%) and then seeded in 6-well culture plates. After 24 h, the α-MEM was replaced with serum-free medium containing M-CSF (25 ng/mL) and RANKL (30 ng/mL). The cells were then incubated for 48 h. (A and B) For the concentration gradient study, 0, 10, 20, 50, and 100 ng/mL OPG were added in the presence of M-CSF and RANKL to various groups of cells. OPG treatment was sustained for 30 min. The control cells (#) were treated with M-CSF and RANKL for another 30 min (C and D). For the time gradient study, 100 ng/mL OPG were added in the presence of M-CSF (25 ng/mL) and RANKL (30 ng/mL) to the different groups of cells. OPG treatment was sustained for 15, 30, 60, and 120 min. The control cells (#) were treated with M-CSF (25 ng/mL) and RANKL (30 ng/mL) without OPG for another 15 min. At each indicated period, TRAP and RANK mRNA expression levels were measured with real-time PCR. Results are expressed as the mean ± SEM. **p < 0.01 and *p < 0.05 vs. the control groups (#).

Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 31172373, 31372495, 31372450, 31302154), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions and Research Fund for the Doctoral Program of Higher Education of China (20113250110003).
References
1. Aeschlimann D, Evans BAJ. The vital osteoclast: how is it regulated? Cell Death Differ. 2004; 11:S5–S7.

2. Boyce BF, Yao Z, Xing L. Osteoclasts have multiple roles in bone in addition to bone resorption. Crit Rev Eukaryot Gene Expr. 2009; 19:171–180.

3. Boyce BF, Yao Z, Zhang Q, Guo R, Lu Y, Schwarz EM, Xing L. New roles for osteoclasts in bone. Ann N Y Acad Sci. 2007; 1116:245–254.

4. Bridgham JT, Johnson AL. Characterization of chicken TNFR superfamily decoy receptors, DcR3 and osteoprotegerin. Biochem Biophys Res Commun. 2003; 307:956–961.

5. Chamoux E, Houde N, L'Eriger K, Roux S. Osteoprotegerin decreases human osteoclast apoptosis by inhibiting the TRAIL pathway. J Cell Physiol. 2008; 216:536–542.

6. Chen J, He JQ, Zhen SY, Huang LQ. OPG inhibits gene expression of RANK and CAII in mouse osteoclast-like cell. Rheumatol Int. 2012; 32:3993–3998.

7. Chen X, Zhu G, Jin T, Gu S, Xiao H, Qiu J. Cadmium induces differentiation of RAW264.7 cells into osteoclasts in the presence of RANKL. Food Chem Toxicol. 2011; 49:2392–2397.

8. Choi HJ, Park YR, Nepal M, Choi BY, Cho NP, Choi SH, Heo SR, Kim HS, Yang MS, Soh Y. Inhibition of osteoclastogenic differentiation by Ikarisoside A in RAW 264.7 cells via JNK and NF-κB signaling pathways. Eur J Pharmacol. 2010; 636:28–35.

9. Cuetara BLV, Crotti TN, O'Donoghue AJ, McHugh KP. Cloning and characterization of osteoclast precursors from the RAW264.7 cell line. In Vitro Cell Dev Biol Anim. 2006; 42:182–188.

10. Feng X. Regulatory roles and molecular signaling of TNF family members in osteoclasts. Gene. 2005; 350:1–13.

11. Fu YX, Gu JH, Wang ST, Wang Y, Zhao HY, Liu W, Tong XS, Liu ZP. Characteristics comparison of osteoclasts induced by M-CSF and RANKL. Chinese J Vet Sci. 2013; 33:94–97.
12. Furukawa M, Takaishi H, Takito J, Yoda M, Sakai S, Hikata T, Hakozaki A, Uchikawa S, Matsumoto M, Chiba K, Kimura T, Okada Y, Matsuo K, Yoshida H, Toyama Y. IL-27 abrogates receptor activator of NF-κB ligand-mediated osteoclastogenesis of human granulocyte-macrophage colony-forming unit cells through STAT1-dependent inhibition of c-Fos. J Immunol. 2009; 183:2397–2406.

14. Gray AW, Davies ME, Jeffcott LB. Generation and activity of equine osteoclasts in vitro: effects of the bisphosphonate pamidronate (APD). Res Vet Sci. 2002; 72:105–113.

15. Grigoriadis AE, Kennedy M, Bozec A, Brunton F, Stenbeck G, Park IH, Wagner EF, Keller GM. Directed differentiation of hematopoietic precursors and functional osteoclasts from human ES and iPS cells. Blood. 2010; 115:2769–2776.

16. Gu JH, Liu JD, Shen Y, Liu ZP. Effects of RANKL, osteoprotegerin, calcium and phosphorus on survival and activation of Muscovy duck osteoclasts in vitro. Vet J. 2009; 181:321–325.

17. Hakeda Y, Kobayashi Y, Yamaguchi K, Yasuda H, Tsuda E, Higashio K, Miyata T, Kumegawa M. Osteoclastogenesis inhibitory factor (OCIF) directly inhibits bone-resorbing activity of isolated mature osteoclasts. Biochem Biophys Res Commun. 1998; 251:796–801.

18. Henriksen K, Leeming DJ, Byrjalsen I, Nielsen RH, Sorensen MG, Dziegiel MH, Martin TJ, Christiansen C, Qvist P, Karsdal MA. Osteoclasts prefer aged bone. Osteoporos Int. 2007; 18:751–759.

19. Hofbauer LC, Neubauer A, Heufelder AE. Receptor activator of nuclear factor-κB ligand and osteoprotegerin: potential implications for the pathogenesis and treatment of malignant bone diseases. Cancer. 2001; 92:460–470.

20. Hou L, Hou J, Yao J, Zhou ZL. Effects of osteoprotegerin from transfection of pcDNA3.1(+)/chOPG on bioactivity of chicken osteoclasts. Acta Vet Scand. 2011; 53:21.

21. Itonaga I, Sabokbar A, Sun SG, Kudo O, Danks L, Ferguson D, Fujikawa Y, Athanasou NA. Transforming growth factor-β induces osteoclast formation in the absence of RANKL. Bone. 2004; 34:57–64.

22. Kobayashi Y, Udagawa N, Takahashi N. Action of RANKL and OPG for osteoclastogenesis. Crit Rev Eukaryot Gene Expr. 2009; 19:61–72.

23. Kwan Tat S, Padrines M, Théoleyre S, Heymann D, Fortun Y. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004; 15:49–60.

24. Lee JW, Kobayashi Y, Nakamichi Y, Udagawa N, Takahashi N, Im NK, Seo HJ, Jeon WB, Yonezawa T, Cha BY, Woo JT. Alisol-B, a novel phyto-steroid, suppresses the RANKL-induced osteoclast formation and prevents bone loss in mice. Biochem Pharmacol. 2010; 80:352–361.

25. Lee SK, Goldring SR, Lorenzo JA. Expression of the calcitonin receptor in bone marrow cell cultures and in bone: a specific marker of the differentiated osteoclast that is regulated by calcitonin. Endocrinology. 1995; 136:4572–4581.

26. Lemay S, Lebedeva TV, Singh AK. Inhibition of cytokine gene expression by sodium salicylate in a macrophage cell line through an NF-κB-independent mechanism. Clin Diagn Lab Immunol. 1999; 6:567–572.

27. Liu H, Zhang R, Ko SY, Oyajobi BO, Papasian CJ, Deng HW, Zhang S, Zhao M. Microtubule assembly affects bone mass by regulating both osteoblast and osteoclast functions: stathmin deficiency produces an osteopenic phenotype in mice. J Bone Miner Res. 2011; 26:2052–2067.

28. Nakamura I, Takahashi N, Jimi E, Udagawa N, Suda T. Regulation of osteoclast function. Mod Rheumatol. 2012; 22:167–177.

29. Nakayama T, Mizoguchi T, Uehara S, Yamashita T, Kawahara I, Kobayashi Y, Moriyama Y, Kurihara S, Sahara N, Ozawa H, Udagawa N, Takahashi N. Polarized osteoclasts put marks of tartrate-resistant acid phosphatase on dentin slices-a simple method for identifying polarized osteoclasts. Bone. 2011; 49:1331–1339.

30. Oguro A, Kawase T, Orikasa M. NaF induces early differentiation of murine bone marrow cells along the granulocytic pathway but not the monocytic or preosteoclastic pathway in vitro. In vitro Cell Dev Biol Anim. 2003; 39:243–248.

31. Price CP, Kirwan A, Vader C. Tartrate-resistant acid phosphatase as a marker of bone resorption. Clin Chem. 1995; 41:641–643.

32. Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997; 89:309–319.

33. Tang CH, Chang CS, Tan TW, Liu SC, Liu JF. The novel isoflavone derivatives inhibit RANKL-induced osteoclast formation. Eur J Pharmacol. 2010; 648:59–66.

35. Vincent C, Kogawa M, Findlay DM, Atkins GJ. The generation of osteoclasts from RAW 264.7 precursors in defined, serum-free conditions. J Bone Miner Metab. 2009; 27:114–119.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download