Abstract
Prebiotics modulate microbial composition and ensure a healthy gastrointestinal tract environment that can prevent colon cancer development. These natural dietary compounds are therefore potential chemopreventive agents. Thirty Sprague-Dawley rats (4 months old) were experimentally treated with procarcinogen dimethylhydrazine to induce colon cancer development. The rats were randomly assigned to three groups: a control group (CG), a group treated with dimethylhydrazine (DMH), and a group given DMH and inulin, a prebiotic (DMH+PRE). The effects of inulin on the activities of bacterial glycolytic enzymes, short-chain fatty acids, coliform and lactobacilli counts, cytokine levels, and cyclooxygenase-2 (COX-2) and transcription nuclear factor kappa beta (NFκB) immunoreactivity were measured. Inulin significantly decreased coliform counts (p < 0.01), increased lactobacilli counts (p < 0.001), and decreased the activity of β-glucuronidase (p < 0.01). Butyric and propionic concentrations were decreased in the DMH group. Inulin increased its concentration that had been reduced by DMH. Inulin decreased the numbers of COX-2- and NFκB-positive cells in the tunica mucosae and tela submucosae of the colon. The expression of IL-2, TNFα, and IL-10 was also diminished. This 28-week study showed that dietary intake of inulin prevents preneoplastic changes and inflammation that promote colon cancer development.
Colorectal cancer (CRC) is a major cause of cancer-related mortality among both men and women worldwide [11]. The risk factors of CRC include age, family history, a history of inflammatory bowel diseases (IBD) including ulcerative colitis and Crohn's disease, and exposure to environmental and dietary procarcinogens [2]. Although the precise process and mechanism underlying colonic carcinogenesis is understood, present therapies including surgery, chemotherapy, radiotherapy, and molecular-targeting therapy are still limited for advanced tumors. Multistage tumorigenesis in the colon with its sequence of accompanying chronic inflammation, genetic and molecular signaling, and metabolic pathways alterations provides an attractive model for investigating the potential of dietary substances for preventing and controlling colon cancer through chemopreventive strategies [16,17]. Chemoprevention is defined as the use of natural dietary compounds and/or synthetic substances that can delay, prevent, or even reverse the development of adenomas as well as the progression from adenoma to carcinoma. The ultimate goal of chemoprevention using natural dietary compounds is to reduce CRC incidence by interfering with development pathways in tumor cells that promote CRC growth and metastases. In this regard, prebiotics as dietary natural compounds that improve intestinal function and ensure a healthy gastrointestinal tract environment have attracted a great deal of interest. Dietary modulation of intestinal microflora by prebiotics can help prevent the development of chronic disesases. The goal of the presented study was to obtain information about the effect of the prebiotic inulin on the activities of bacterial glycolytic enzymes, short-chain fatty acids (SCFAs), coliform and lactobacilli counts, cytokine levels, and expression of the chemopreventive markers cyclooxygenase-2 (COX-2) and transcription nuclear factor kappa beta (NFκB) in colon tissues from rats with dimethylhydrazine (DMH)-induced colon cancer.
All animal experiments were conducted in accordance with the principles outlined in Law No. 23/2009 of the Slovak Republic for the Care and Use of Laboratory Animals, and were approved by the Ethical Committee of the Faculty of Medicine of P. J. Šafarik University in Košice (The Slovak Republic). Thirty male and female Sprague-Dawley rats (Central Vivarium Faculty of Medicine, P. J. Šafárik University, The Slovak Republic) 4 months old with a mean initial body weight of 378.73 ± 81.25 g were housed with a 12-h light/dark cycle. The room was maintained at 21℃ ± 1℃ with 50% to 60% humidity. The rats were randomly assigned to the following three groups: a control group (CG), a DMH treatment group (DMH), and a group treated with both DMH and inulin (DMH+PRE). Rats in the CG group were fed only conventional feed (Biofer, The Slovak Republic). Two weeks after beginning the experiment, rats in the DMH and DMH+PRE groups were treated with DMH (Merck, Germany) at a dose of 21 mg/kg body weight (subcutaneously) five times at weekly intervals. The experimental diets were consumed during the entire. Rats in the DMH group received conventional feed combined with DMH injection. Rats in the DMH+PRE group received conventional feed supplemented with inulin (oligofructose-enriched inulin, Orafti Synergy1; BENEO, Belgium) at a dose 80 g/kg of conventional feed and DMH injection. All animals had free access to water. Dietary intake was recorded daily and animal weights were measured weekly. The food intake efficiency rate (FER) was estimated with the formula: FER = (final weight-initial weight)/ average dietary intake [10]. After 28 weeks of consuming the experimental diets, the animals were euthanized under anesthesia (100 mg ketamin/kg body weight with 15 mg xylazin/kg body weight, intraperitoneal). Blood samples were then drawn by cardiac puncture to measure interleukin concentrations. Feces were recovered for microbial and biochemical analysis, and tissue samples from the jejunum and colon were taken.
Microbial analyses (total lactobacilli and coliform) of the fecal samples were carried out after completion of the experiment. Feces (1 g) were placed in a sterile polyethylene Stomacher Lab Blenders bag (Seward, France) with 9 mL sterile 0.9% NaCl. A series of 10-fold dilutions (10-2 to 10-8) was made with the same sterile diluent. From each dilution, 0.1-mL aliquots were spread onto two selective McConkey agar plates (Merck, Germany) for coliforms and Rogosa agar plates (Biocar Diagnostic, France) for lactobacilli. The plates for lactobacilli culturing were maintained under anaerobic conditions (BD GasPaK; Becton, Dickinson and Company, USA) and incubated at 37℃ for 48 h. Plates used for coliform culturing were incubated aerobically at 37℃ for 16~18 h. Viable counts are expresses as the log 10 of colony forming units (CFU) per gram of feces. The colonic pH was measured using a pH meter kit with a pH electrode SP 1DT (Merck).
The activity of bacterial enzymes was measured in fresh caecal digesta taken after the experiment by determining the rate of p- or o-nitrophenol by the nitrophenylglucosides as previously described by Juskiewicz et al. [12]. The following substrates (Sigma Aldrich, USA) were used: p-nitrophenyl-α-D-glucopyranoside (for α-glucosidase; α-GLU), p-nitrophenyl-β-D-glucopyranoside (for β-glucosidase; β-GLU), p-nitrophenyl-α-D-galactopyranoside (for α-galactosidase; α-GAL), o-nitrophenyl-β-D-galactopyranoside (for β-galactosidase; β-GAL), and p-nitrophenyl-β-D-glucuronide (for β-glucuronidase; β-GLUCUR). The reaction contained 0.3 mL of a substrate solution of nitrophenylglucoside (5 mM) and 0.2 mL of a 1 : 10 (v/v) dilution of the fecal samples in 100 mM phosphate buffer (pH 7.0) centrifuged at 10,000 g for 15 min at 4℃. Incubation was carried out at 37℃ for 10 min, and p- or o-nitrophenol was and and after addtion of 2.5 mL of 0.25 M cold sodium carbonate was measured absorbation at 400 nm. A measurement unit of enzymatic activity is expressed as µmol of p-nitrophenol per min per gram of digesta. SCFAs were analyzed in the caecal digesta using gas chromatography (6890 Plus; Hewlett Packard, USA) and expressed as mmol/100 mL of wet cecal digesta.
Serum levels of interleukin (IL)-2 and IL-10 were measured by commercially ELISA kits (eBiosciences, USA). The concentration of TNFα in the serum was also determined with an ELISA kit (RayBio, USA) according to the manufacturer's directions. A segment of the jejunum was taken, flushed with ice-cold PBS (pH 7.2), and opened longitudinally. The frozen scraped jejunal mucosa was homogenized in PBS and centrifuged at 12,000 g for 20 min at 4℃. The supernatant was collected and the levels of TNFα, IL-2, and IL-10 were measured with the ELISA kits.
The distal portion of the colon was removed, fixed in 10% buffered formalin, and embedded in paraffin. Histological sections were stained by hematoxilin-eosin for determination of inflammation and preneoplastic lesion in the colon. Immunohistochemical staining for COX-2 and NFκB was performed on paraffin sections 4~5-µm-thick using a commercial horseradish peroxidase-3, 3'diaminobenzidine (HRP-DAB) Cell and Tissue Staining Kit (R&D Systems, UK). COX-2 and NFκB expression in the tunica mucosae and tela submucosae of the colon was observed by incubating the sections with specific primary anti-COX-2 (rabbit polyclonal, diluted 1 : 500; Abcam, UK) and anti-NFκB (rabbit polyclonal, diluted 1 : 100; MBL International, USA) antibodies for 2 h at 37℃. Immunoreactivity was visualized in the form of a dark brown precipitate formed after enzymatic conversion of DAB by HRP at the sites of antigen localization. COX-2 and NFκB expression was expressed as the number of positive cells in 1,000 µm2 of colon tissue.
At the end of the experiment, mean body weight of the rats in the CG group had increased by 21.43%. Mean body weight had increased by 19.89% in the DMH group and by 28% in the DMH+PRE group. The highest FER (p < 0.05) was observed for the DMH+PRE group relative to the CG and DMH groups. For the CG group, the coliform count was 6.17 ± 0.56 log10 CFU/g and the lactobacilli count was 8.99 ± 0.45 log10 CFU/g. DMH injection slightly increased the coliform counts and decreased lactobacilli counts (6.34 ± 0.25 and 8.78 ± 0.37, respectively). Inulin significantly decreased the coliform counts (5.96 ± 0.22 log10 CFU/g; p < 0.01) and significantly increased the lactobacilli counts (9.38 ± 0.29 log10 CFU/g; p < 0.001) compared to the DMH group.
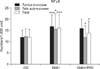
Changes in glycolytic enzyme activity for all groups are summarized in Table 1. DMH increased the activity of β-glucuronidase and α-glucosidase (p < 0.01) while decreasing the activity of α-galactosidase (p < 0.01). Inulin treatment significantly decreased β-GLUCUR (p < 0.01) activity and increased that of α-GAL (p < 0.01). Butyric and propionic concentrations were decreased in the DMH group (p < 0.001), and inulin increased the concentration of these two compounds (p < 0.01; Table 2). Furthermore, inulin significantly decreased the expression of the proinflammatory cytokines IL-2 and TNF-α, and stimulated the production of regulatory IL-10 in the jejunal mucosa. Similar tendeny of changes in proinflammatory cytokines and regulatory IL-10 were recorded in the serum (Fig. 1). The numbers of COX-2- and NFκB-positive cells in the tunica mucosae and tela submucosae of the colon are presented in Figs. 2 and 3, respectively.
Inulins are a group of non-digestible oligosaccharides known as a fructans [8,9]. In order to be effective, a prebiotic must escape digestion in the upper gastrointestinal tract so that it can be released in the lower tract and used by the beneficial microorganisms, mainly bifidobacteria and lactobacilli, in the colon. The colonic microflora is involved in the etiology of colorectal cancer. Although the precise bacterial types associated with colorectal cancer risk have not been elucidated, it is clear that some bacterial groups (lactobacilli and bifidobacteria) have much lower activities of enzymes that can generate carcinogens compared to other gut microflora components such as coliforms, clostridia, and Bacteroides. In our study, the number of lactobacilli was the highest in the group treated with the prebiotic inulin. Coliform counts were significantly reduced in the same group of rats while those of animals in the DMH group were the highest.
Increased activities of α-GAL and β-GAL seen after treatment with a prebiotic could be attributed to increased levels of lactobacilli and decreased by coliforms [15]. The activity of β-GLUCUR is believed to be a biomarker of an increased risk of neoplasm and is also perceived as harmful due to the associated release of carcinogens from hepatically derived glucuronic acid conjugates [13]. Additionally, β-GLUCUR is a critical factor for the enterohepatic circulation of drugs and other foreign compounds [13].
Undigested food is fermented in the colon by the microbiota and gives rise to various microbial metabolites [21,23]. SCFAs including acetic, propionic, and butyric acid are the principal metabolites produced. However, most of the literature has focused on butyrate and (to a lesser extent) on acetate. Consequently, the potential physiological and pathological effects of propionic acid have long been underestimated [14]. Butyrate causes apoptosis, reduces metastasis of colon cell lines, and protects against genotoxic carcinogens [20]. Elevated butyric acid concentrations observed in the DMH+PRE group is in accordance with experimental animal models which revealed that inulin-type fructans have anticarcinogenic properties [18]. In the colon, propionic acid is produced by the fermentation of polysaccharides, oligosaccharides, long-chain fatty acids, protein, peptides, and glycoprotein precursors by the anaerobic colonic microbiota although in quantitative terms indigested carbohydrates represent the major source for propionic acid production.
It is now well established that the gastrointestinal tract is in a permanent state of low-grade inflammation. The intake of dietary fiber, which is the primary substrate for propionic acid production, has been associated with a reduction in low-grade inflammation and intestinal inflammatory pathogenesis [7]. Propionic acid has moderate inhibitory activity on cyclooxygenase. Given the concentration of propionic acid in the colonic intestine, a direct anti-inflammatory effect of propionic acid via cyclooxygenase is to be expected. This likely represents a mechanism for the reduction of low-grade mucosal inflammation by prebiotic diets. Furthermore, both prebiotic diet consumption and cyclooxygenase inhibition are associated with reduced incidence of colorectal cancer. Propionic acid-dependent inhibition of cyclooxygenase may be an implicated effect as well [22]. This observation is strongly supported by Comalada et al. [3] who discovered that bacterium-derived SCFAs seem to be directly responsible for the anti-carcinogenic effects of pre/pro-biotic supplementation in preclinical models of colon cancer.
COX-2 is important enzyme that mediates inflammatory processes. Enhanced expression of COX-2 but not COX-1 has been observed in many different types of tumors and transformed cells [19]. In the current study, the highest total numbers of COX-2-positive cells were found in colon tissue from the DMH group. The levels of IL-2 and TNFα followed a similar pattern (Fig. 1). Increased concentrations of proinflammatory cytokines and low levels of regulatory cytokines in the mucosal immune system and at the systemic level are associated with chronic inflammation and tumor progression. Since inflammation is closely linked to tumor formation, substances with potent anti-inflammatory activities are anticipated to exert chemopreventive effects against carcinogenesis, particularly during the developmental stage. In the current investigation, inulin decreased the numbers of COX-2-positive cells as well as the concentration of IL-2 and TNFα, and stimulated IL-10 production, thus demonstrating its anti-inflammatory activity and immune-enhancing effect.
Bakhle demonstrated that NFκB is involved in regulation of COX-2. Several chemopreventive phytochemicals have been shown to inhibit COX-2 by blocking improper NFkB activation [1]. The transcriptional activity of NFκB is regulated via an elaborate series of intracellular signal transduction events in response to external stimuli such as mitogens and inflammatory cytokines [5]. In addition to its central role in mediating inflammation, NFκB is important for controlling cell proliferation, oncogenesis, and cell transformation [5]. Furthermore, this factor has drawn much interest as an attractive therapeutic target for novel anti-inflammatory and immunomodulatory drugs [4]. In the experimental DMH group, the total numbers of COX-2-positive and NFκB-positive cells in colon tissue were significantly increased. Inulin decreased the total numbers of cells positive for COX-2 and NFκB.
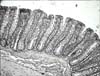
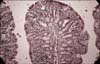
No histopathological changes were observed in the colon tissue sections from the CG group (Fig. 4). Lieberkühn crypts had an adequate depth with a large number of goblet cells. Lymphocytic cellulization was adequate. In some sections of colon tissue, focal thinning without any signs of overgrowth of lymphoid tissue was observed. In the area of thinning were present crypts with reduced length and plenty of goblet cells. In the DMH group, the formation of colorectal cancer was not induced but non-specific chronic inflammation (chronic catarrahal colitis) was found (Fig. 5). The intestinal tissue contained lymphatic follicles surrounding fragment crypts (crypts with a disrupted structure) with their immersion into lymphoid tissue (cavitation crypts in lymphoid tissue) is a precondition for adenoma in case captured bearing is subjected to prolonged inflammation. Chronic non-specific inflammation was more pronounced in the colon distalis where artificial separation of the tunica mucosae from the tela submucosae was observed. In the tunica mucosae layer, we found a cluster of morphologically altered cells showing preneoplastic changes. In the DMH+PRE group, fewer signs of inflammation and preneoplastic changes were seen, particularly in the colon distalis. In the tunica submucosae, reduced Lieberkühn crypts with a narrow lumen were also present. The goblet cells were enlarged and more goblet cells in the colon distalis were noted compared to the colon proximalis. Femia et al. [6] showed that arabinoxylan-oligosaccharides reduce the number of preneoplatic lesions in the colon of rats within 13 weeks. Our study lasting for 28 weeks demonstrated that dietary intake of inulin by rats prevented preneoplastic changes and inflammation, suggesting that this prebiotic exerts a chemopreventive effect on colon cancer.
Figures and Tables
Fig. 1
Serum and jejunal cytokines IL-2, IL-10 and TNF alpha in the CG group, DMH group and DMH+PRE group. Statistical significance is result of comparison between CG/DMH and DMH/DMH+PRE. *p < 0.05 and ***p < 0.001.

Fig. 2
Expression of the numbers of COX-2-positive cells in the tunica mucosae and tela submucosae of the colon, and total numbers of COX-2-positive cells in the colon. Statistical significance is result of comparison between CG/DMH and DMH/DMH+PRE. **p < 0.01 and ***p < 0.001.

Fig. 3
Expression of the numbers of NFκB-positive cells in the tunica mucosae and tela submucosae of the colon, and total numbers of COX-2-positive cells in the colon. Statistical significance is result of comparison between CG/DMH and DMH/DMH+PRE. *p < 0.05 ***p < 0.001.
Acknowledgments
This work was supported by the Agency of the Slovak Ministry of Education for the Structural Funds of the European Union under ITMS 26220120058 (20%) and ITMS 26220220104 (80%).
References
1. Bakhle YS. COX-2 and cancer: a new approach to an old problem. Br J Pharmacol. 2001; 134:1137–1150.

2. Benson AB 3rd. Epidemiology, disease progression, and economic burden of colorectal cancer. J Manag Care Pharm. 2007; 13:6 Suppl C. S5–S18.

3. Comalada M, Bailón E, de Haro O, Lara-Villoslada F, Xaus J, Zarzuelo A, Gálvez J. The effects of short-chain fatty acids on colo epithelial proliferation and survival depend on the cellular phenotype. J Cancer Res Clin Oncol. 2006; 132:487–497.

4. Chabot-Fletcher M. Transcription factor NFκB: an emerging anti-inflammatory drug target. Pharmacol Commun. 1996; 8:317–324.
5. Chen F, Castranova V, Shi X, Demers LM. New insights into the role of nuclear factor-κB, a ubiquitous transcription factor in the iniciation of diseases. Clin Chem. 1999; 45:7–17.

6. Femia AP, Salvadori M, Broekaert WF, François IEJA, Delcour JA, Courtin CM, Caderni G. Arabinoxylan-oligosaccharides (AXOS) reduce preneoplastic lesions in the colon of rats treated with 1,2-dimethylhydrazine (DMH). Eur J Nutr. 2010; 49:127–132.

7. Galisteo M, Duarte J, Zarzuelo A. Effects of dietary fibers on disturbances clustered in the metabolic syndrome. J Nutr Biochem. 2008; 19:71–84.

8. Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995; 125:1401–1412.

9. Gibson GR, Probert HM, Loo VJ, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004; 17:259–275.

10. Henriques VT, Dias CMGC, Franceschini SCC, Sabarense CM, Costa NMB, Leite JIA, Peluzio MCG. Omega-3 fatty acids reduce the development of preneoplatic lesions. Rev Nutr Campinas. 2009; 22:237–244.
11. Janakiram NB, Rao CV. Molecular markers and targets for colorectal cancer prevention. Acta Pharmacol Sin. 2008; 29:1–20.

12. Juskiewicz J, Zdunczyk Z, Wroblewska M, Oszmianski J, Hernandez T. The responce of rats to feeding with diets. Food Res Int. 2002; 35:201–205.
13. Juśkiewicz J, Wróblewska M, Jaroslawska J, Baliński P, Matusevičius P, Zduńczyk P, Biedrzycka E, Zduńczyk Z. Effects of inulin supplemented to cellulose-free or cellulose-rich diets on caecal environment and biochemical blood parameters in rats. J Anim Feed Sci. 2009; 18:709–722.

14. Al-Lahham SH, Peppelenbosch MP, Roelofsen H, Vonk RJ, Venema K. Biological effects of propionic acids in humans; metabolism, potential applications and underlying mechanisms. Biochim Biophys Acta. 2010; 1801:1175–1183.

15. Lay C, Sutren M, Lepercq P, Juste C, Rigottier-Gois L, Lhoste E, Lemée R, Le Ruyet P, Doré J, Andrieux C. Influence of Camembert consumption on the composition and metabolism of intestinal microbiota: A study in human microbiota-associated rats. Br J Nutr. 2004; 92:429–438.

16. Mathew A, Peters U, Chatterjee N, Kulldorff M, Sinha R. Fat, fiber, fruits, vegetables, and risk of colorectal adenomas. Int J Cancer. 2004; 108:287–292.

17. Pan MH, Ho CT. Chemopreventive effects of natural dietary compounds on cancer development. Chem Soc Rev. 2008; 37:2558–2574.

18. Pool-Zobel BL, Sauer J. Overview of experimental data on reduction of colorectal cancer risk by inulin-type fructans. J Nutr. 2007; 137:2580S–2584S.

19. Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995; 55:3785–3789.
20. Scharlau D, Borowicki A, Habermann N, Hofmann T, Klenow S, Miene C, Munjal U, Stein K, Glei M. Mechanisms of primary cancer prevention by butyrate and other products formed during gut flora-mediated fermentation of dietary fibre. Mutat Res. 2009; 682:39–53.

21. Schley PD, Field CJ. The immune-enhancing effect of dietary fibres and prebiotics. Br J Nutr. 2002; 87:Suppl 2. S221–S230.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download