Introduction
Tubular epithelium of the kidney is composed of highly polarized cells attached to one other. Cell polarity and integrity are maintained by specialized cell-cell junctions including tight junctions (zonula occludens), adherens junctions (zonula adherens), and desmosomes (macula adherens) [5]. Numerous molecules regulate renal epithelial cell junctions and abnormal distribution of adhesion proteins has been implicated in the pathogenesis of various renal diseases [6,11,15,19,22].
Cadherin family members are transmembrane proteins of the adherens junction and desmosome. Previous studies have shown that a variety of cadherins including E-, N-, P-, K-, R-, OB-, VE-, and Ksp-cadherin are present in the kidney [18,22,24,25]. Among these, classical cadherins such as E- and N-cadherin are the most abundant. Renal expression of E-cadherin appears to be nephron segment-specific in different species of animals. E-cadherin is expressed in all tubular segments of mouse and rabbit kidney [20,25]. In rat, dog, monkey, and human kidney, E-cadherin is abundant in the distal tubules but is not expressed in the proximal tubules (or is present only at very low levels) [18,21]. Instead, N-cadherin is the predominant cadherin in the proximal tubule [18,21]. E-cadherin may be important for assessment and management of kidney transplant. Pigs are good candidates for kidney transplant because they are similar in anatomical structure to humans. However, the expression and localization of renal E-cadherin in these animals is lacking.
Materials and Methods
Animals and tissue preservation
All animal studies were approved by the Ewha Womans University Institutional Animal Care and Use Committee (Korea). Male three-breed pigs (25~30 kg; Han-Lim Lab. Animal, Korea) derived from Large White, Landrace, Duroc and Sprague-Dawley rats (200~250 g; Orient Bio, Korea) were used for all experiments. The animals were anesthetized by inhalation of 1.5% isoflurane (Abbott Laboratories, Canada). The kidneys were preserved by in vivo perfusion through the renal artery. The animals were initially perfused briefly with PBS (298 mOsmol/kg H2O, pH 7.4) to rinse away the blood. For Western blot analysis, the right kidneys were excised after the renal artery was clamped. For immunocytochemical studies, the left kidneys were then perfused with a 0.01 M periodate-0.075 M lysin-2% paraformaldehyde (PLP; Sigma-Aldrich, Germany) solution for 5 min. After perfusion, the kidneys were removed and cut into 1- to 2-mm-thick slices that were fixed by immersion in the PLP solution overnight at 4℃. The tissue sections (50-µm-thick) were cut transversely with a vibratome 1,000 plus section system (Intracel, UK) and processed for electron microscopic immunocytochemistry using a horseradish peroxidase preembedding technique. Some tissues were dehydrated in a graded series of ethanol and embedded in paraffin.
Antibodies
Purified mouse IgG2a specific for human E- and N-cadherin (catalog numbers 610181 and 610921; BD Transduction Labs, USA) was used for this study. Additionally, anti-aquaporin 1 (catalog number AB2219; Chemicon, USA), anti-Tamm-Horsfall protein (catalog number sc-19554; Santa Cruz Biotechnology, USA), anti-calbindin D28k (catalog number AB1778; Chemicon), and anti-H+-ATPase (catalog number sc-20943; Santa Cruz Biotechnology) were used to identify the proximal tubule, thick ascending limb, distal convoluted tubule, and collecting duct, respectively [1,2,17,23].
Light and electron microscopic immunocytochemistry
Fifty-µm tissue sections were processed for immunocytochemistry using an indirect preembedding immunoperoxidase method as previously described [7,8]. All sections were washed three times with 50 mM NH4Cl in PBS for 15 min at room temperature. Before incubation with the primary antibody, the sections were pretreated with a graded series of ethanol followed by incubation for 3 h at room temperature with PBS containing 1% bovine serum albumin (BSA), 0.05% saponin, and 0.2% gelatin (solution A; Sigma-Aldrich, Germany). The tissue sections were then incubated overnight at 4℃ with antibodies against E-cadherin (1 : 200) and N-cadherin (1 : 50) diluted in 1% BSA-PBS (solution B; Sigma-Aldrich). Control sections were incubated in solution B without primary antibody. After several washes with solution A, the sections were incubated for 2 h at room temperature in peroxidase-conjugated donkey anti-mouse IgG Fab fragment (Jackson ImmunoResearch Laboratories, USA) diluted 1 : 100 in solution B. The tissues were first rinsed in solution A and the rinsed in 0.05 M Tris buffer (pH 7.6). To detect antibody binding, the sections were incubated at room temperature in 0.1% 3,3'-diaminobenzidine (DakoCytomation; Dako, USA) in 0.05 M Tris buffer for 5 min. Next, H2O2 was added at a final concentration of 0.01% and the incubation was continued for 10 min at room temperature. After washing with 0.05 M Tris buffer, the sections were dehydrated in a graded series of ethanol.
The 50-µm-thick vibratome sections through the entire kidney were embedded in poly/Bed-812 resin (Polysciences, USA) between polyethylene vinyl sheets and examined by light microscopy. Additionally, 1-µm semi-thin sections were examined by light microscopy (Olympus BX51; Olympus, Japan). 50-nm ultrathin sections were then cut, stained with 0.4% lead citrate (SPI-Chem, USA), and photographed with a transmission electron microscope (H-7650; Hitachi, Japan).
Double immunocytochemistry
To identify the exact localization of E-cadherin, double immunoperoxidase techniques as previously described [9,10,13]. For the first immunoperoxidase procedure, the protocol for single labeling was performed as described above. After the diaminobenzidine reaction, the sections were washed in PBS, incubated in 3% H2O2 for 30 min, and then in protein blocking solution (DakoCytomation, Dako) for 30 min at room temperature. The sections were treated for 1 h with primary antibodies (anti-aquaporin 1, 1 : 1,000; anti-Tamm-Horsfall protein, 1 : 500; anti-calbindin D28k, 1 : 1,000; and anti-H+-ATPase, 1 : 200), washed in PBS, incubated with secondary antibodies (Jackson ImmunoResearch Laboratories, USA), and then washed with PBS at room temperature. Vector SG (Vector Laboratories, USA) was used as the chromogen to produce a blue stain easily distinguished from the brown tint produced by the diaminobenzidine. The sections were washed in Tris-HCl buffer, dehydrated with graded ethanol and xylene, mounted, and viewed by light microscopy.
Immunoblot analysis
For immunoblot analysis, proteins from rat and pig kidneys were extracted as previously described [8,13]. The kidneys were homogenized in lysis buffer containing 20 mM Tris-HCI, 1% Triton X-100, 150 mM sodium chloride, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 0.02% sodium azide, 1 mM EDTA, 10 µM leupeptin, and 1 mM phenylmethylsulfonyl fluoride (Sigma-Aldrich). The homogenate was centrifuged at 3,000 g for 20 min at 4℃. After measuring the protein concentration in the supernatant using the Coomassie method (Pierce, USA), the samples were loaded (30 µg/lane) onto sodium dodecyl sulfate-polyacrylamide gels and underwent electrophoresis under reducing conditions. The proteins were then transferred to nitrocellulose membranes (Sigma-Aldrich) by electroblotting. reduce nonspecific binding, the membranes were blocked in 5% nonfat dried milk (Sigma-Adrich) for 30 min at room temperature, and then incubated for 24 h at 4℃ with affinity-purified anti-E-cadherin (1 : 1,000). After three washes with TBS, the blots was incubated with peroxidase-conjugated donkey anti-mouse IgG (1 : 1,000; Jackson ImmunoResearch Laboratories) for 2 h at room temperature. Antibody binding was visualized using an enhanced chemiluminescence system (Amersham Life Science, UK) on a LAS-3000 imager (Fujifilm, Japan) at room temperature.
Results
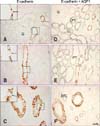
In normal adult pig kidneys, E-cadherin expression was observed from the outer cortex to the inner medulla. The area containing E-cadherin was proportionately greater in the medulla than in the cortex (Figs. 1A~C). E-cadherin immunostaining was present in the basolateral plasma membrane domain of the tubular epithelial cells (Figs. 1A~C, insets) as previously described [3,12,21,25]. Expression of this protein was not detected in glomeruli or blood vessels. Double immunolabeling with aquaporin 1 revealed that E-cadherin was not expressed in the proximal tubule or descending thin limb but high levels were present in the distal tubule of the pig kidney (Figs. 1D~F).
Further studies were performed to characterize the distal tubule, which includes the thick ascending limb, distal convoluted tubule, and collecting duct [14]. Antibodies against Tamm-Horsfall protein, calbindin D28k, and H+-ATPase were used to identify the thick ascending limb, distal convoluted tubule, and collecting ducts, respectively [1,2,23]. Double immunolabeling demonstrated that E-cadherin was expressed in the calbindin D28k-positive distal convoluted tubule and H+-ATPase-positive collecting ducts in the renal cortex as previously reported in rats (Figs. 2A and C). In contrast to the rat kidney, E-cadherin was not expressed at detectable levels in the Tamm-Horsfall protein-positive thick ascending limb of either the renal cortex or medulla of the pig kidney (Figs. 2B and D). N-cadherin was predominantly expressed in the basolateral plasma membranes of the proximal tubule (Fig. 3A). N-cadherin labeling was not detected in the distal tubule or collecting duct that expressed E-cadherin (Fig. 3B).
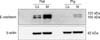
Immunoblots of kidney protein extracts from rats and pigs showed two bands of the same size (approximately 120 and 105 kDa). Renal expression of E-cadherin was relatively lower in pigs compared to rats. Additionally, E-cadherin expression in both the rats and pigs was much higher in the renal medulla than the cortex (Fig. 4).
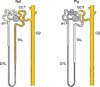
Electron microscopy immunocytochemistry revealed that E-cadherin was localized in the basolateral plasma membranes and basal infoldings of the collecting duct. E-cadherin immunostaining was not detected in the thick ascending limb (Fig. 5). Differences between the expression patterns of rat and pig E-cadherin are presented in Fig. 6.
Discussion
The present study provides the first detailed description of E-cadherin expression in pig kidney. E-cadherin expression was observed in the distal convoluted tubule and collecting duct. This protein was not found in the proximal tubule or thick ascending limb.
There is a general agreement that renal E-cadherin expression is nephron segment-specific in different animal species. E-cadherin is expressed at uniform levels along the entire nephron of mouse and rabbit kidney whereas it is expressed predominantly in the distal tubule of rats, dogs, monkeys, and humans [18,20,21,25]. The distal tubule includes the thick ascending limb, distal convoluted tubule, and collecting duct [14]. Previous study on rat kidney have shown that E-cadherin is expressed in most distal tubules including the distal convoluted tubule, collecting ducts, and possibly portions of the thick ascending limb [21]. In the present investigation, we confirmed that E-cadherin was localized in the thick ascending limb in the rat kidney using a specific marker (Tamm-Horsfall protein). We also demonstrated that E-cadherin was expressed in the distal convoluted tubule and collecting duct of pig kidney but was not present in the thick ascending limb. These findings may explain the relatively lower levels of E-cadherin expression in the pig kidney compared to the rat observed in the current study (Fig. 4). E-cadherin is expressed in the human thick ascending limb [12]. It has not been conclusively determined whether E-cadherin expression is present in dog and monkey thick ascending limb.
Interestingly, E-cadherin is strongly expressed in the collecting duct. E-cadherin is important for maintaining cell junctions in kidney epithelial cells [4,22]. Renal ischemia-reperfusion injury damages the collecting duct and disruption of intercellular junctions plays a critical role in the pathophysiology of this condition [4,10,13,15]. Pigs are currently thought to be the best candidates for kidney donation because they are physiologically similar to humans. During renal xenotransplantation, ischemia and subsequent reperfusion of donor kidneys can lead to delayed graft function. Maintaining the structural integrity of the tubular epithelial cells is probably a significant factor for preventing chronic graft dysfunction [16]. Disruption of cadherin localization is also involved in chemical-induced nephrotoxicity and renal cell carcinoma development [11,22,24]. Toxic reagents can damage the collecting duct cells and alter the localization of cadherins [22]. Chromophobe renal cell carcinoma and renal oncocytoma originate from the collecting duct whereas clear cell renal carcinoma and papillary renal carcinoma arise from the proximal tubule [9,26].
In the present study, we demonstrated that E-cadherin is a major adhesion factor expressed in the distal convoluted tubule and collecting duct of the pig kidney. In contrast to the human and rat, E-cadherin expression was not detected in the thick ascending limb in the pig kidney. Kidney is composed of distinct cell types which show different sensitivities to toxic injury. Differences in E-cadherin expression may provide an important clue in the search for the molecular pathogenesis and cancer etiology.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download