Abstract
Tendinitis of the superficial digital flexor tendon (SDFT) is a significant cause of lameness in horses; however, recent studies have shown that stem cells could be useful in veterinary regenerative medicine. Therefore, we isolated and characterized equine umbilical cord blood mesenchymal stem cells (eUCB-MSCs) from equine umbilical cord blood obtained from thoroughbred mares during the foaling period. Horses that had tendinitis of the SDFT were treated with eUCB-MSCs to confirm the therapeutic effect. After eUCB-MSCs transplantation, the core lesion in the SDFT was found to decrease. These results suggest that transplantation using eUCB-MSCs could be another source of cell treatment.
Tendinitis of the superficial digital flexor tendon (SDFT) is a significant cause of lameness and often a career-ending event in thoroughbred horses because of its high incidence, prolonged recovery period, and high rate of recurrence [5,15]. Afflicted horses are prone to distal limb injury due to hyperextension of the metacarpal joint during racing or riding; thus, the SDFT suffers the highest frequency of injury in race horses [10]. After injury, the equine SDFT repairs via a process of fibrosis, but the scar tissue that forms is functionally deficient compared to that of the normal tendon [2,3,19]. Moreover, recurrences are related to the production of a collagen fibril matrix and regeneration of tendon tissue that is smaller in diameter and of inferior quality to normal tendon tissue [20]. However, equine mesenchymal stem cells (MSCs) have recently been studied as a potential new clinical resource to resolve these problems.
MSCs have been derived from various equine tissues, including bone marrow [1,11,17], adipose tissue [11,18], peripheral blood [13], umbilical cord blood [12], umbilical cord matrix [9], and amniotic fluid [16]. Recent studies of equine MSC implantation have focused on MSCs derived from adipose tissue and bone marrow [8,14,15,17]. However, to date, the use of equine umbilical cord blood mesenchymal stem cells (eUCB-MSCs) for treating naturally or experimentally occurring SDFT injury has not been reported. Here, we present the first case report of a clinical application of eUCB-MSCs in naturally induced tendinitis and a description of eUCB-MSC surface markers.
Equine umbilical cord blood (n = 6) was obtained from thoroughbred mares during the foaling period in a stall at a private horse farm in the Republic of Korea. The collected blood was transported to the laboratory in tubes treated with EDTA anticoagulant. Prior to analysis, blood was diluted 1:1 with phosphate buffered saline (Cellgro; Mediatech, USA), after which a density gradient using Ficoll-Paque (GE Healthcare, USA) was performed to collect the buffy coat layer. Mononucleated cells were seeded into T75 cell culture flasks (Nunc/Nalge, USA) at 5 × 106 cells/mL. Three days after the cells were seeded, they were transferred to new flasks and half the amount of Dulbeco's Modified Eagle's Medium (low glucose DMEM; LG-DMEM; Gibco BRL, USA) was exchanged. The adhered cells were then trypsinized to maintain passage 7 days after the primary cells were seeded.
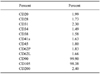
A cell proliferation assay was performed as previously described, with some modifications [16]. Briefly, the estimated growth efficiency and proliferation potential of the eUCB-MSCs were determined based on the total cumulative population doubling level (CPDL) using the formula CPDL = ln (Nf/Ni) ln 2, where Ni is the initial seeding cell number, Nf is the final harvest cell number, and ln is the natural log. Cells were stained for flow cytometry with specific antibodies according to the manufacturer's protocol (BD Biosciences, USA). For analysis, the following mouse antihuman CD markers were used: CD20, CD28, CD31, CD34, CD38, CD41a, CD45, CD62L, CD62P, CD90, CD105, and CD200 (BD Biosciences). Analysis was performed using a FACS Calibur instrument (BD Biosciences) and the Cell Quest Pro (BD Biosciences) software.
To confirm differentiation ability, eUCB-MSCs were treated with osteogenic differentiation medium containing ascorbic acid 2-phosphate (50 µM), dexamethasone (100 nM), β-glycerophosphate (10 mM) (Sigma-Aldrich, USA) and 10% fetal bovine serum (FBS) in LG-DMEM. Briefly, eUCB-MSCs (1 × 105) were plated in triplicate on 6-well plates, after which the cells were maintained in osteogenic differentiation medium for 3 weeks. Following differentiation, the cells were washed with PBS and fixed with ice-cold 70% ethanol for 1 h at 4℃ for subsequent Alizarin Red S staining (40 mM; pH 4.2; Sigma-Aldrich), which was conducted for 10 min at room temperature. Following staining, the cells were rinsed with distilled water [16]. eUCB-MSCs were treated with adipogenic differentiation medium containing dexamethasone (1 µM), indomethacin (60 µM), 3-isobutyl-1-metyl-xanthine (500 µM) and insulin (5 µg/mL) (Sigma-Aldrich) in 10% FBS in LG-DMEM. After the cells reached 80~90% confluency, they were treated with adipogenic differentiation medium for 3 weeks. Following differentiation, the cells were fixed by incubation in 10% formalin for at least 1 hr and then rinsed with 60% isopropanol prior to incubation in freshly diluted Oil Red O for 10 minutes [6]. Next, eUCB-MSCs were treated with chondrogenic differentiation medium and seeded (5 × 105 cells) in a 15 mL polypropylene tube and centrifuged to a pellet. The pellets were then cultured in 1 mL of chondrogenic differentiation medium (Lonza, USA) and incubated for 3 weeks. After differentiation, the pellets were embedded in paraffin and cut into 3 µm sections that were stained with toluidine blue and Alcian blue-PAS to detect chondrogenesis following standard protocols [16].
The characterized eUCB-MSCs were used for equine tendinitis therapy in clinical cases. Overall, six clinical cases ranging in age from 3~5 years (all male thoroughbred race horses) were assessed in this study. SDFT was diagnosed by a clinical veterinarian via ultrasonographic examination. The interval between diagnosis and implantation was 2~4 weeks. None of the six horses implanted with allologous eUCB-MSCs exhibited adverse reaction due to cell allograft. Defining the optimum time for injecting MSCs was difficult because of differences in lesion core size, lesion length, horse age, and horse management. However, sufficient time was allowed for adequate angiogenesis and granulation tissue to form, which would more likely be of benefit than an earlier hemorrhagic and inflammatory lesion. We determined the injection time based on clinical signs such as fever, hemorrhage, edema, and pain to avoid the early inflammatory period.
The SDFT core lesions were monitored by repeated ultrasonographic examinations before and after eUCB-MSC injection. Ultrasonographic examinations with a 15-MHz, 40-mm linear probe (Mylab 30; Esaote, Italy) were conducted in the transverse and longitudinal planes to evaluate the SDFT. The tendonitis core lesion was prepared aseptically for infection. Briefly, an aliquot of 2 × 107 MSCs resuspended in 10 mL PBS was slowly injected into the SDFT core lesions. Ultrasonographic guidance was used to ensure that the needle entered the core lesion from the lateral aspect of the SDFT. A total of 20 injection sites were designated from the proximal SDFT region to the distal SDFT region and the 0.5 mL contents of each syringe (26 gage needle) were injected into the center or lateral aspect of each tendon.
After injecting the eUCB-MSCs, the horses were rested in a private stall without exercise for 3~4 weeks. The horses then received rehabilitation that consisted of hand walking, walking, trotting, and cantering for 3~6 months before returning to racing.
The eUCB-MSCs showed fibroblast-like and spindle morphology, which is typical of MSCs, and adhered to the plastic culture flask surface (Fig. 1A). We calculated and measured the cell population via the CPDL to detect the proliferation ability of eUCB-MSCs. To accomplish this, eUCB-MSCs (5 × 104 cells/well) were seeded in a 6-well culture plate and subcultured after 5~7 days. This procedure was repeated until passage 18 to measure the CPDL. A stable increase in cell growth was observed (Fig. 1B), which is characteristic of stem cells. Additionally, stem cells have self-renewal ability, which is associated with continuous and steady cell proliferation; therefore, these results demonstrate that the isolated eUCB-MSCs were capable of self-renewal.
MSCs display a distinctive cell surface antigen pattern, including CD90 and CD105, while antigens that are not typically found on MSCs include CD11b, CD14, CD19, CD79a, CD34, CD45, and HLA-DR [16]. Flow cytometry analysis for 12 CD markers (CD20, CD28, CD31, CD34, CD38, CD41a, CD45, CD62L, CD62P, CD90, CD105, and CD200) was conducted using eUCB-MSCs at passage 5 to determine whether they displayed the MSC phenotype (Table 1). The eUCB-MSCs showed positive CD90 and CD105 expression. CD90, which is also known as Thy-1, is a marker for various types of stem cells, including hepatic stem cells, keratinocyte stem cells, endometrial stem cells, and MSCs [19,20]. CD105, which is also known as SH2, is a well-known MSC marker [4]. Other markers such as those expressed by immune cells (CD20, CD28, CD38, CD62L, and CD200), endothelial cells (CD31 and CD62P), hematopoietic cells (CD34), and platelets (CD41a) were not expressed. These results showed that the eUCB-MSCs had an immunophenotype similar to that of a typical MSC.
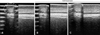
Positive and strong Alizarin Red S staining was detected (Figs. 1C and D); however, the cells were negative for Alizarin Red S under basal culture conditions (Figs. 1E and F). The cells were also stained with Oil Red O to detect fatty droplets in the adipogenic differentiation investigation. Under differentiation conditions, fatty droplets were observed and positively stained with Oil Red O (Figs. 1G and H). Black arrows indicate that fatty droplets were stained with Oil Red O (Fig. 1H). The basal culture medium was used as a negative control, and no staining was observed under the control conditions (Figs. 1I and J). Pellet formation was detected at the bottom of the polypropylene tube in the chondrogenic differentiation study. The morphology of the pellets was ovoid and opaque. The white arrow indicates pellet formation (Fig. 1K). The pellet stained positively with toluidine blue (Fig. 1L).
The eUCB-MSCs-treated horses recovered from their clinical condition and had a good SDFT ultrasound image after the rehabilitation program. Ultrasonographic examination was repeated at 1, 2, 4, 8, and 12 weeks. The size of the SDFT core lesion decreased dramatically in the transversal ultrasonographic images 1 month after the eUCB-MSC injection (Fig. 2), while the tendons were almost completely repaired 3 months after the MSC injection. The core SDFT lesion on the longitudinal ultrasonographic image was significantly different before and after injection of the eUCB-MSCs. Ultrasonographic lesion severity was determined by the lesion cross sectional surface area (CSA) and length influence prognosis [7]. Thus, we used the CSA category grouping to evaluate transplantation efficacy. According to Genovese et al. [7], the CSA category of SDFT is divided into six groups. Among these, category 4 has an eventual 80% failure rate, while categories 5 ~ 6 have a higher failure rate [7] and there is no advantage or disadvantage observed with changes in exercise in categories 4~6. These observations indicate that CSA categories 4 ~ 6 have a poor prognosis and require retirement from racing or long-term treatment.
In the present study, all SDFTs of the horses presented with CSA category 5 before transplantation. One month after transplantation, all horses without clinical signs presented as CSA category 1. Two horses were included in the failed group, and four horses were included in the successful group of the Genovese et al. [7] grouping method. CSA category 5 had a 90% failure rate in Genovese et al. [7], whereas we observed only a 33% failure rate in this category. Overall, these results indicate that clinical application of eUCB-MSCs has some value, and that they could be useful in regenerative medicine and a source of stem cells in horses.
Figures and Tables
 | Fig. 1Primary culture, cumulative population doubling level (CPDL), and differentiation of equine umbilical cord blood mesenchymal stem cells (eUCB-MSCs). (A) Phase contrast images of eUCB-MSCs. Cell morphology showed a spindle and fibroblast-like structure that was similar to that of human mesenchymal stem cells. (B) Cell growth curve of eUCB-MSCs. CPDL was measured from passages 3 to 18. (C~F) Osteogenic differentiation. Alizarin Red S staining was conducted after 3 weeks of osteogenic induction. Osteogenic differentiated cells (C, D) were grown in osteogenic induction medium and differentiated cells stained strongly with Alizarin Red S. Control cells (E, F) were grown in normal low glucose DMEM with 10% FBS and showed no staining with Alizarin Red S. (G~J) Adipogenic differentiation. Oil Red O staining was conducted after 3 weeks of adipogenic induction. Adipogenic differentiated cells (G, H) were grown in adipogenic induction medium and differentiated cells were stained with Oil Red O. The black arrows indicate stained red fat droplets. Control cells (I, J) were grown in normal low glucose DMEM with 10% FBS and no staining with Oil Red O was observed. (K, L) Chondrogenic differentiation. After 3 weeks of chondrogenic induction, pellets aggregated into a round shape. (K) Pictures of the round shaped chondrogenic pellet. A pellet formed at the bottom of the 15 mL polypropylene tube. The white arrow indicates a pellet. (L) Toluidine blue staining of chondrogenic pellets. Scale bars = 50 µm (A, D, F, H and J), and 100 µm (L). |
Acknowledgments
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST, 2010-0020265).
References
1. Arnhold SJ, Goletz I, Klein H, Stumpf G, Beluche LA, Rohde C, Addicks K, Litzke LF. Isolation and characterization of bone marrow-derived equine mesenchymal stem cells. Am J Vet Res. 2007; 68:1095–1105.

2. Crevier-Denoix N, Collobert C, Pourcelot P, Denoix JM, Sanaa M, Geiger D, Bernard N, Ribot X, Bortolussi C, Bousseau B. Mechanical properties of pathological equine superficial digital flexor tendons. Equine Vet J Suppl. 1997; 23:23–26.

3. Dahlgren LA, Mohammed HO, Nixon AJ. Temporal expression of growth factors and matrix molecules in healing tendon lesions. J Orthop Res. 2005; 23:84–92.

4. Domimici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, Deans RJ, Keating A, Prockop DJ, Horwitz EM. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8:315–317.

5. Dyson SJ. Medical management of superficial digital flexor tendonitis: a comparative study in 219 horses (1992-2000). Equine Vet J. 2004; 36:415–419.

6. Gargett CE. Identification and characterisation of human endometrial stem/progenitor cells. Aust N Z J Obstet Gynaecol. 2006; 46:250–253.

7. Genovese R, Longo K, Berthold B, Jorgensen J. Quantitative sonographic assessment in the clinical management of superficial digital flexor injures in thoroughbred racehorses. AAEP Proceedings. 1997; 43:285–290.
8. Godwin EE, Young NJ, Dudhia J, Beamish IC, Smith RKW. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet J. 2012; 44:25–32.

9. Hoynowski SM, Fry MM, Gardner BM, Leming MT, Tucker JR, Black L, Sand T, Mitchell KE. Characterization and differentiation of equine umbilical cord-derived matrix cells. Biochem Biophys Res Commun. 2007; 362:347–353.

10. Kasashima Y, Takahashi T, Smith RKW, Goodship AE, Kuwano A, Ueno T, Hirano S. Prevalence of superficial digital flexor tendonitis and suspensory desmitis in Japanese thoroughbred flat racehorses in 1999. Equine Vet J. 2004; 36:346–350.

11. Kisiday JD, Kopesky PW, Evans CH, Grodzinsky AJ, McIlwraith CW, Frisbie DD. Evaluation of adult equine bone marrow- and adipose-derived progenitor cell chondrogenesis in hydrogel cultures. J Orthop Res. 2008; 26:322–331.

12. Koch TG, Heerkens T, Thomsen PD, Betts DH. Isolation of mesenchymal stem cells from equine umbilical cord blood. BMC Biotechnol. 2007; 7:26.

13. Koerner J, Nesic D, Romero JD, Brehm W, Mainil-Varlet P, Grogan SP. Equine peripheral blood-derived progenitors in comparison to bone marrow-derived mesenchymal stem cells. Stem Cells. 2006; 24:1613–1619.

14. de Mattos Carvalho A, Alves ALG, de Oliveira PGG, Álvarez LEC, Amorim RL, Hussni CA, Deffune E. Use of adipose tissue-derived mesenchymal stem cells for experimental tendinitis therapy in equines. J Equine Vet Sci. 2011; 31:26–34.

15. Nixon AJ, Dahlgren LA, Haupt JL, Yeager AE, Ward DL. Effect of adipose-derived nucleated cell fractions on tendon repair in horses with collagenase-induced tendinitis. Am J Vet Res. 2008; 69:928–937.

16. Park SB, Seo MS, Kang JG, Chae JS, Kang KS. Isolation and characterization of equine amniotic fluid-derived multipotent stem cells. Cytotherapy. 2011; 13:341–349.

17. Smith RKW, Korda M, Blunn GW, Goodship AE. Isolation and implantation of autologous equine mesenchymal stem cells from bone marrow into the superficial digital flexor tendon as a potential novel treatment. Equine Vet J. 2003; 35:99–102.

18. Vidal MA, Kilroy GE, Johnson JR, Lopez MJ, Moore RM, Gimble JM. Cell growth characteristics and differentiation frequency of adherent equine bone marrow-derived mesenchymal stromal cells: adipogenic and osteogenic capacity. Vet Surg. 2006; 35:601–610.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download