Pseudorabies virus (PRV), which belongs to the family Herpesviridae, subfamily Alphaherpesvirinae, and genus Varicellovirus, is the causative agent of Aujeszky's disease (AD), more commonly known as pseudorabies (PR) [4]. PRV can infect livestock and wild animals, resulting in increased morbidity and mortality [1-3]. In general, PRV mainly infects pigs at various production phases, such as causing high mortality in newborn piglets and abortion in pregnant sows, and results in significant economic losses for the pork industry.
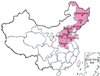
Beginning in October 2011, a severe PRV outbreak occurred on several pig farms and spread rapidly to most of northern China, affecting more than nine provinces, including Henan and Hebei, and autonomous cities such as Beijing City (Fig. 1). The infected animals presented with multiple clinical signs, including high fever (usually 40~42℃), depression, anorexia, cough, shivering, diarrhea, and systemic neurological symptoms. Infected pigs were emaciated and weak in appearance and ultimately died. Unexpectedly, growing and finishing pigs also died during this epidemic period, with a reported mortality rate of 10~30%.
A total of 540 serum samples from 20 pig farms were collected from nine provinces and autonomous cities. Pigs on the 20 pig farms had been vaccinated with PR vaccine according to procedures recommended by the manufacturers. These vaccines were constituted with different strains, most of which were PR Bartha-K61. PRV antibodies were detected using the IDEXX gE Ab enzyme-linked immunosorbent assay (ELISA) kit (IDEXX Laboratories, Westbrook, USA), and the specific gE antibody could differentiate vaccinated from wild type virus infections. The results of ELISA showed a total positive rate of 51.9% (280/540). The highest positive rate was 94.2% (49/52) in samples from Henan province, and the lowest positive rate was 16.7% (10/60) in samples from Heilongjiang province, indicating severe PRV infections throughout these regions.
Twenty clinical porcine brain samples from different provinces were sent to our laboratory for diagnosis. DNA and RNA were extracted from the tissue homogenates and classic swine fever virus (CSFV), porcine reproductive and respiratory syndrome virus (PRRSV), and PRV were detected by reverse transcription polymerase chain reaction (RT-PCR) or PCR. Only PRV was found in these samples (18 of 20 samples were PRV positive). Then, following successful isolation of PRV in porcine kidney (PK-15) cells, we observed obvious cytopathological effects, characterized by cell congregation, contraction, and brushing off at passage 3. PCR analysis and DNA sequencing were performed in order to confirm identification of the viruses as PRV.
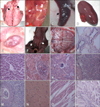
PR strain HN1201, which was recently isolated in Henan province, was used for examination of the pathogenicity in 35-day-old PRV-free piglets in a secure, biosafety level 2 (P2) facility. All procedures were approved by the ethics committee of the China National Research Center for Veterinary Medicine. Each of the piglets (N = 4) received intranasal challeng with 108.0 50% tissue culture infecting dose of the isolated virus propagated in PK-15 cells. The piglets were insulated in separate rooms throughout the experiment (two weeks) for daily observation of clinical signs, such as cough, sneeze, dyspnea, abnormal behavior, and increased rectal temperature. Tissue samples of brain, lungs, heart, liver, kidneys, spleen, tonsil, and lymph nodes of deceased piglets were obtained for histopathological examination and viral isolation. The clinical signs expressed by the challenged pigs were consistent with those of pigs isolated from farms, including high fever, depression, anorexia, cough, shivering, diarrhea and systematic neural symptoms, and so forth. We observed the death of one piglet on day 5 post-infection, two on day 6, and one on day 8. PRV was re-isolated from the brain tissues of the challenged piglets. In postmortem and histopathological examinations of dead piglets, multiple lesion sites were observed in several organs (Fig. 2). In summary, evidence from investigation of epidemic history, serum testing, viral isolation, laboratory challenges, and histopathological examinations confirmed that a recent viral epidemic in northern China was caused by PRV infection in pig populations.
Despite a lack of evidence, emergence of the new viral strain was probably caused by a recombination event resulting from different vaccine strains, as many different strains are used for vaccination against PR on many Chinese pig farms. These strains include Bartha, Bartha-K61, Bucharest, BUK, HB-98, and SA215, among others. In this study, PR outbreaks occurred on farms where pigs were immunized with PR vaccine, which implied that the PR vaccine did not provide effective protection against wild PR infection. Understanding the molecular mechanisms driving PRV virulence in northern China is important and further experimental verification will be required in the near future.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download