Abstract
This study was conducted to establish an in vitro maturation (IVM) system by selection of efficient porcine serum during porcine in vitro production. To investigate the efficient porcine serum (PS), different types of PS [newborn pig serum, prepubertal gilt serum (PGS), estrus sow serum, and pregnancy sow serum] were used to supplement IVM media with or without gonadotrophin (GTH) and development rates of parthenogenetic activation (PA) and in vitro fertilization (IVF) embryos were then compared. The maturation rates of the PGS group was significantly higher when GTH was not added. Additionally, during development of PA embryos without GTH, the PGS group showed significantly higher cleavage and blastocyst formation rates. Moreover, the cleavage rates of IVF embryos were significantly higher in the PGS group, with no significant differences in the blastocyst formation. However, when GTH was supplemented into the IVM media, there were no significant differences among the four groups in the cleavage rates, development rates of the blastocyst, and cell number of the blastocyst after PA and IVF. In conclusion, PGS is an efficient macromolecule in porcine IVM, and GTH supplementation of the IVM media is beneficial when PS is used as macromolecule, regardless of its origin.
Oocyte maturation is one of the important stages for successful production of in vitro fertilization (IVF) and somatic cell nuclear transfer embryos [1]. Because in vitro maturation (IVM) is not as efficient as that of in vivo, many studies are needed to determine the optimal culture media compositions needed to maximize the maturation rates [1,6,20]. As a result, researchers have been developing maturation media supplemented with porcine follicular fluid (pFF) or serum as macromolcules. Although pFF has been used in most studies of porcine embryo development, the properties of pFF have not been maintained and they may be a source of infection because follicular fluid could only be obtained from abbatoir-derived ovaries.
Heat inactivated serum added to medium as a protein supplement may play an essential role in the IVM system [28]. Serum as macromolecules has been shown to be necessary for the oocyte maturation, and fetal bovine serum (FBS), estrus calf serum (ECS), pro-estrus calf serum (PECS), steer serum, and maternal serum have been used in bovine IVM [12,28,29,37]. Although the oocyte maturation rate was not different after IVM in media supplemented with these different sera, when compared with that of FBS supplemented media, ECS supplemented IVM showed differing quality for most layers and increased cleavage rates of IVF embryos [29]. Moreover, 62.9% maturation rates were observed when FBS was used as a supplement in the IVM media, which was significantly lower than that of oocytes in media with bovine serum obtained at D0 (estrus), D1 (metestrus), D10 (diestrus) or D20 (proestrus) [29]. In addition, D20 and/or D0 bovine serum may contain factors that increase the developmental competence of oocytes during IVM [37]. Similarly, the most high fertilization rate was shown when PECS was added to the media at the standing estrus [28].
In vivo oocyte maturation occurs in the follicular fluid. Because equilibrium is established during follicular growth, the fluid has components similar to serum. However, follicular fluid also contains secretions from the ovarian follicle, which reflect the follicular synthetic activity [12,15]. Moreover, among other components, a variety of sex steroids is contained in follicular fluid at concentrations signaificantly higher than that of serum [10,16,17]. However, while the importance of sex hormones is well known in the ovary, the influence of the follicular fluid on the oocyte development is not well understood. Changes in the steroid content of follicular fluid according to the stage of follicles (size, atretic phase, and growing phase) and the follicular fluid characteristics are affected by the donor's physiological condition [16]. During oocytes maturation, an orderly sequence of changes, which in the follicular steroid hormone concentrations, may affect the oocyte directly by producing changes in Ca2+ release or indirectly via granulosa cells [3,24,34]. Additionally, it has been shown that steroid hormones are involved in meiotic arrest of oocytes and important in the acquisition of fertilization competence in the oocytes [12,24,30,37]. However, the effects of estradiol on oocyte maturation, ovulation and embryonic development seems to be species dependent [5,8,9,13,33]. In fact, it has been reported that a detrimental effect of estradiol supplementation was detrimental on cytoplasmic maturation in the porcine oocytes, while that improved the quality of IVM oocytes in human and bovine IVF [11,33,37].
Gonadotropin (GTH) supplementation in the IVM media has been shown to increase the fertilization rates and embryonic development after IVF [22,23,37]. Moreover, it was revealed that D20 serum had a higher concentration of luteinizing hormone (LH) that can significantly increase the maturation rates in IVM [37]. As mentioned above, ECS had a significantly greater effect on bovine IVM and cleavage and development to blastocysts after IVF when compared with FBS. Although some studies have used porcine serum (PS) and FBS instead of pFF for IVM media supplementation [35], there have been no reports about the effects of donor stage of porcine serum.
The objectives of the present study were to examine the effects of different stages of porcine serum supplemented porcine IVM media with and without additional GTH supplementation on maturation and development to the blastocyst stage after parthenogenetic activation (PA) and IVF.
All chemicals were purchased from Sigma Chemical Company (USA) in this study if it is not stated. The basic medium in the oocyte maturation was tissue culture medium-199 with Earle's salts, L-glutamine, 2.2 g/L sodium bicarbonate, 0.8 mM L-cysteine, 0.4 mM Na-pyruvate, 1.13 mM kanamycin, 10 ng/mL epidermal growth factor and 1 µg/mL insulin (Invitrogen, USA). The changes of components in the defined basic medium were made according to each aspect to be examined in oocyte maturation. The IVF medium was modified Tris-buffered medium [2] and that for embryonic development was North Carolina State University (NCSU)-23 [25].
PS was prepared by centrifugation of venous blood from pigs in the each of four donors (newborn piglets; 5 weeks from birth, prepubertal gilt; 3 months from birth, estrous sow; 1 year from birth, and pregnant sow; 1 year from birth) at 4,000 × g for 20 min, filtered through a 1.2, 0.45, and 0.2 µm syringe filter (Gelman Sciences, USA) sequentially and stored in aliquots at -80℃ until use.
Porcine ovaries were taken from prepubertal gilts and sows at a local slaughterhouse and transported to the laboratory quickly. The ovaries were collected from gilts and placed in 0.9% saline at 30~37℃ for less than 3 h, after which they were washed twice in sterile saline. Cumulus-oocytes complexes (COCs) were aspirated from follicles with diameter of 3~8 mm using an 18 G needle attached to a 10 mL syringe. The follicular contents were then pooled in a 15 mL conical tube for 5 min, after which the supernatant was discarded. COCs in the sediment were washed 3 times and only COCs with homogenous cytoplasm and more than two layers of cumulus cells were collected for IVM.
Approximately 50 COCs were placed to each well of a 4-well multidish (Nunc; ThermoFisher Scientific, USA) containing 500 µL of IVM medium with GTH including 10 IU/mL pregnant mare's serum gonadotropin (PMSG) (Intervet, The Netherlands) and 10 IU/mL human chorionic gonadotropin (hCG) (Intervet) at 39℃ and 5% CO2 in a humidified atmosphere. After maturation for 22 h, COCs were transferred to IVM medium without PMSG and hCG and cultured for more 22 h. After IVM, COCs were denuded by treatment of 0.1% (w/v) hyaluronidase (H-3506) and repeated pipetting.
The sperm-rich fractions of Landrace were collected from a pig artificial insemination center (DARBY AI center, Korea). Semen was slowly chilled to 15℃ over 2 h after collection. One volume of sperm was then diluted with two volumes of HulsenbergVIII extender [27] and centrifuged at 15℃ for 10 min at 400 × g, after which the supernatant solution was poured off. The pellet was then resuspended with the first diluted solution [BF5; 52.3 mM TES, 16.5 mMTrizma base, 177.8 mM D (+)-glucose, 5 mL/L OEP (or vs. paste), 0.02 g/L gentamycin sulfate and 200 mL/L egg yolk] and diluted to provide 1.0 × 109 sperm/mL. Next, semen was cooled to 5℃ over a 1h period and one volume of BF5 + 2% (v/v) glycerol (G-9012) (second diluted solution) was mixed with one volume of cooled semen. Straws (IMV Technologies, France) were immediately filled with 2.5 mL of semen, after which a needle holder was used to seal the ends of the straws. Next, the straws were horizontally placed on an aluminum rack and set into a tank containing liquid nitrogen (LN) 4 cm above the LN for 20 min, after which they were immersed in the LN for storage. Frozen sperm were then examined for motility (percentage of total motile sperm evaluated) and viability (strength of the sperm tail beating on a 0~5 scale) after thawing. Following examination, only sperm with greater than 60% motility and a vigor score above 3 were used.
For electrical activation, matured oocytes with the first polar body after denuding were equilibrated for 5 min in activation solution (280 mM mannitol, 0.1 mM CaCl2·2H2O and 0.05 mM MgCl2·6H2O) and then transferred to the same activation solution on the electric chamber consisting of two electrodes 1 mm apart. Single pulse of 1.2 kV/cm was exposed to oocytes for 60 µs on a BTX Electro cell manipulator 2001 (BTX, USA). After activation by DC pulse, oocytes were washed twice with TLH-PVA and then transferred into NCSU-23 supplemented with 5 µg/mL cytochalasin B (C-6762) for 5~6 h for post-activation at 39℃ under humidified conditions and 5% CO2.
Oocytes were randomly selected after denuding from each treatment and washed 3 times in IVF medium, after which 15 oocytes were placed into each of 45 µL drop of the same medium that had been covered with warm mineral oil. Next, a frozen semen pellet was thawed and washed 3 times by centrifugation at 350 × g for 4 min in sperm washing solution. After sperm washing, the sperm pellet was resuspended and diluted in IVF medium. After appropriate dilution, 5 µL sperm suspension was added to 45 µL drops that contained oocytes with final sperm concentration of 2.0 × 106/mL. Finally, oocytes and spermatozoa were coincubated for 6 h at 39℃ under 5% CO2 in humidified air.
After fertilization or PA, groups of ten oocytes was then cultured in 30 µL NCSU-23 droplets containing 4 mg/mL BSA at 39℃ under humidified conditions and 5% CO2. The cleavage rates of blastocyst formation rates were examined on day 2 and day 7 after fertilization or activation, respectively. To investigate the developmental competence of the blastocysts, blastocysts were then stained with Hoechst 33342 and cell number was counted.
In this study, the effects of porcine serum from different stages with or without additional GTH supplementation on oocyte maturation and blastocyst formation were evaluated after PA and IVF.
In the porcine IVM, the effects of different stage porcine serum supplementation without GTH were evaluated. Approximately 50 COCs were transferred to each well of a 4-well multidish containing 500 µL of IVM medium with 4 different stage porcine serum without GTH. After culturing for 22 h, COCs were transferred to IVM medium without PMSG and hCG and then cultured for another 22 h.
Following maturation, the effects of different stages of porcine serum were evaluated on nuclear maturation and cleavage rates, blastocyst formation rates, and cell number in the blastocysts after PA and IVF and compared among the four groups.
In IVM, the effects of different stage porcine serum supplementation with GTH were evaluated. Approximately 50 COCs were transferred to each well of a 4-well multidish (Nunc) containing 500 µL of IVM medium with each 4 different stage porcine serum and GTH at 39℃ in a humidified atmosphere of 5% CO2. After culturing for 22 h, COCs were transferred to IVM medium without PMSG and hCG and then cultured for another 22 h.
Following maturation, the effects of different stages of porcine serum were evaluated on nuclear maturation and cleavage rates, blastocyst formation rates, and cell number in the blastocysts after PA and IVF and compared among the four groups.
Maturation rates of porcine oocytes after using different stage PS without GTH for supplementation of maturation media are shown in Table 1. The maturation rates of prepubertal gilt serum (PGS) (-) group were significantly higher than those of pregnant sow serum (PSS) (-) (p < 0.05). The developmental potential of porcine parthenogenetic oocytes matured in media supplemented with different stages of PS without GTH are shown in Table 2. The cleavage rate of the PGS (-) group was significantly (p < 0.05) higher than that of the PSS (-) group, while the development of the blastocyst rate of the PGS (-) group was significantly (p < 0.05) higher than that of the ESS (-) and PSS (-) group. There were no significant differences in the cell number of blastocysts among the four groups.
The developmental potential of porcine oocytes after IVF matured in media supplemented with different stages of PS without GTH are shown in Table 3. The cleavage rate of the PGS (-) group was significantly (p < 0.05) higher than that of the PSS (-) group; however, there were no significant differences among the four groups in the development to blastocyst rates. The cell number of blastocysts of the ESS (-) group was significantly (p < 0.05) higher than that of the NPS (-) and PSS (-) group.
Maturation rates of porcine oocytes after using different stage PS supplementation with GTH used as maturation media are shown in Table 4. The maturation rates of ESS (+) were significantly higher than those of the NPS (+) and PSS (+) group (p < 0.05).
The developmental potential of porcine parthenogenetic oocytes matured in media supplemented with different stages of PS with GTH are shown in Table 5. There were no significant differences among the four groups in the cleavage rates, development to blastocyst rate, and cell number of blastocysts.
The developmental potential of porcine IVF embryos after maturation in media supplemented with different stage PS and GTH are shown in Table 6. There were no significant differences among the four groups in the cleavage rates, development to blastocyst rate, and cell number of blastocysts.
In this study, IVM media was supplemented with four types of PS with and without GTH to investigate what stages of PS were efficient in the development of PA and IVF embryos. In experiment 1, the maturation, cleavage, and blastocyst formation rates of porcine oocytes were higher in the PGS (-) group among the four groups, except for the rate of development to blastocysts after IVF, which did not differ significantly among the four groups. The cell number of blastocysts was only significantly higher in the ESS (-) group after IVF than in the NPS (-) and PSS (-) group. In experiment 2, significant differences were shown only in maturation rates not in the cleavage rates, development rates to blastocyst, and cell number of blastocyst. Moreover, comparison of the overall data from experiment 1 and 2 revealed that developmental competence was significantly higher in the presence of GTH supplementation.
Bovine serum (FBS or ECS) has been used as the main protein source in bovine IVM studies, and ECS had a significant and marked effect on maturation and development to blastocysts when compared with FBS. Moreover, Younis et al. [37] suggested that PECS collected on the day prior to estrus might be more effective in IVM than ECS. However, in the present study, the maturation rates were not significantly higher in the ESS group. Several studies have shown that follicular sex steroids may affect the oocyte maturation [9,26,33]. The estradiol function is expressed via estrogen receptor (ER) in cumulus cells and oocytes during IVM and affects progesterone synthesis by pig follicular cells when such cells are cultured. Since progesterone is one of the major sex steroids after the LH surge, it is needed in the porcine oocyte maturation [9]. However, the estradiol effects on IVM of mammalian oocytes matured in chemically defined medium were variable. The positive estradiol effects on cytoplasmic maturation have been reported in bovine, human, and rabbit oocytes, whereas no effect was observed for sheep oocytes [13,26,33,36,37]. In porcine models, little effect of estradiol on oocyte maturation suggesting that a high concentration of estradiol is only needed in meiotic arrest of oocytes and not for maturation itself [9].
In PA embryos without GTH, the rate of development to blastocysts of the ESS (-) group were significantly lower in those of the PGS (-) group. Moreover, in the IVF embryos cultivated without GTH, there were no significant differences among the four groups in the development rate to blastocyst. These results were similar to those of previous studies in that the incidences of male pronuclear (MPN) formation and embryonic development were significantly lower in oocytes matured with supplementation of estradiol [8,18]. In addition, only progesterone increased subsequent decondensation of sperm head and MPN formation in the oocytes [21]. Moreover, in the PSS (-) group, the maturation and cleavage rates were significantly lower than in the NPS (-) group. These results were in accordance with the previous finding that there were no significant differences among oocytes that matured in the presence of estradiol and progesterone, absence of estradiol and progesterone, and presence of progesterone alone [21]. However, PS was added to the IVM media, which might contain steroid hormones such as estradiol and progesterone. Accordingly, some unknown factors contained within PS may have some direct effects of progesterone on oocyte maturation.
During follicular development, the estrogen concentration in follicular fluid gradually increases in response to FSH stimulation. However, after the ovulatory LH surge or treatment with hCG, the estradiol concentration decreases drastically, after which the concentration of progesterone increases [3,12,14,15]. In fact, GTH supplementation of the IVM medium could be similar effect with in vivo LH surge and may stimulate sex steroid secretion by the COCs. The cumulus cells then respond to GTH by increasing progesterone in vitro [4,8]. In experiment 2, the maturation rates of the medium supplemented with ESS were significantly higher than those of NPS and PSS. However, there were no significant differences in the cleavage rates, development to blastocyst rates, and cell number of blastocysts among the four groups cultivated in media supplemented with GTH. These results were similar to those of a previous study that showed no steroid needs to be added to the medium during oocyte maturation. Moreover, these results do not provide evidence of a specific role of steroids during oocyte maturation or of a beneficial effect of estradiol. It is possible that much of steroids produced by the COCs or contained in PS was sufficient to cause the biological response [8].
Moreover, the maturation rates, cleavage rates, and development to blastocyst rates of PA and IVF embryos with GTH in the IVM media were significantly higher than those without GTH. These results were similar to those of other studies that showed that medium supplemented with EGF and GTH together with estradiol can produce synergetic maturation in vitro in a protein-free maturation system [9,19]. Moreover, D20 serum (proestrus) had a higher concentration of LH in bovines, D 20 serum and added GTH for oocyte maturation promote viability of bovine embryos resulting from IVF [37].
In addition, the significant difference in the nuclear status of in vitro matured oocytes was observed clearly based on morphological differences in COCs. Specifically, COCs attached to the bottom of dish showed earlier nuclear maturation than these of floating [31,32]. Similarly, the COCs of the ESS (-) and PSS (-) group were attached to the bottom of the culture dish and the oocytes were matured more early with dispersed cumulus cells in this study. Conversely, the COCs of NPS (-) and PGS (-) group were attached to the bottom of dish and the oocytes were surrounded by a compact somatic compartment. However, in experiment 2, the COCs of all groups were floating in the maturation medium and the oocytes were surrounded by a light colored fully expanded cumulus mass (unpublished data).
In conclusion, the results of the present study suggest that PGS had a significant and marked effect on the subsequent developmental competence of oocytes when compared with ECS and PSS in the porcine IVP system. However, when medium was supplemented with GTH, the effects of different stages of PS did not differ significantly and were more available than those without GTH. Moreover, in a previous study, the cumulus expansion stimulating factor is not species-specific and the 2nd fraction of PS exhibited a marked expansion of the cumulus cells and active factors [7]. However, clarification of these aspects that affect maturation, cleavage, and development to blastocyst rates and more investigation for optimal IVM conditions with molecular mechanisms involved in the effects of steroid hormones on the oocyte maturation requires further study.
Figures and Tables
Table 1
Comparison of maturation rates of porcine embryos according to origins of porcine serum without gonadotropin

Table 2
Comparison of developmental rates of porcine parthenogenetic activation (PA) embryos according to the origins of porcine serum without gonadotropin

Table 3
Comparison of developmental rates of porcine in vitro fertilization (IVF) embryos according to the origins of porcine serum without gonadotropin
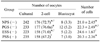
Table 4
Comparison of maturation rates of porcine embryos according to origins of porcine serum with gonadotropin

Acknowledgments
This work was supported by a grant from the Next-Generation BioGreen 21 Program (No. PJ009604), Rural Development Administration, Republic of Korea.
References
2. Abeydeera LR, Day BN. Fertilization and subsequent development in vitro of pig oocytes inseminated in a modified tris-buffered medium with frozen-thawed ejaculated spermatozoa. Biol Reprod. 1997; 57:729–734.

3. Ainsworth L, Tsang BK, Downey BR, Marcus GJ, Armstrong DT. Interrelationships between follicular fluid steroid levels, gonadotropic stimuli, and oocyte maturation during preovulatory development of porcine follicles. Biol Reprod. 1980; 23:621–627.

4. Channing CP, Bae IH, Stone SL, Anderson LD, Edelson S, Fowler SC. Porcine granulosa and cumulus cell properties. LH/hCG receptors, ability to secrete progesterone and ability to respond to LH. Mol Cell Endocrinol. 1981; 22:359–370.

5. Conley AJ, Bird IM. The role of cytochrome P450 17α-hydroxylase and 3β-hydroxysteroid dehydrogenase in the integration of gonadal and adrenal steroidogenesis via the Δ5 and Δ4 pathways of steroidogenesis in mammals. Biol Reprod. 1997; 56:789–799.

6. Coy P, Romar R. In vitro production of pig embryos: a point of view. Reprod Fertil Dev. 2002; 14:275–286.

7. Daen FP, Sato E, Nakayama T, Toyoda Y. Serum factor(s) stimulating cumulus expansion in porcine oocyte-cumulus complexes matured and fertilized in vitro. Cell Struct Funct. 1995; 20:223–231.

8. Dode MAN, Graves C. Involvement of steroid hormones on in vitro maturation of pig oocytes. Theriogenology. 2002; 57:811–821.

9. Dode MAN, Graves CN. Role of estradiol-17β on nuclear and cytoplasmic maturation of pig oocytes. Anim Reprod Sci. 2003; 78:99–110.

10. Eiler H, Nalbandov AV. Sex steroids in follicular fluid and blood plasma during the estrous cycle of pigs. Endocrinology. 1977; 100:331–338.

11. Funahashi H, Day BN. Effects of the duration of exposure to hormone supplements on cytoplasmic maturation of pig oocytes in vitro. J Reprod Fertil. 1993; 98:179–185.

12. Gordon IR. Laboratory Production of Cattle Embryos. 2nd ed. Wallingford: CAB International;2003. p. 126–132.
13. Guler A, Poulin N, Mermillod P, Terqui M, Cognié Y. Effect of growth factors, EGF and IGF-I, and estradiol on in vitro maturation of sheep oocytes. Theriogenology. 2000; 54:209–218.

14. Guthrie HD, Barber JA, Leighton JK, Hammond JM. Steroidogenic cytochrome P450 enzyme messenger ribonucleic acids and follicular fluid steroids in individual follicles during preovulatory maturation in the pig. Biol Reprod. 1994; 51:465–471.

15. Hafez ESE, Hafez B. Reproduction in Farm Animals. 7th ed. Ames: Weily-Blackwell;2006. p. 68–81.
16. Iwata H, Inoue J, Kimura K, Kuge T, Kuwayama T, Monji Y. Comparison between the characteristics of follicular fluid and the developmental competence of bovine oocytes. Anim Reprod Sci. 2006; 91:215–223.

17. Leroy JLMR, Vanholder T, Delanghe JR, Opsomer G, Van Soom A, Bols PEJ, Dewulf J, de Kruif A. Metabolic changes in follicular fluid of the dominant follicle in high-yielding dairy cows early post partum. Theriogenology. 2004; 62:1131–1143.

18. Li Q, Niwa K, Hunter MG. Effects of 17β-estradiol on in vitro maturation of pig oocytes in protein-free medium. J Reprod Dev. 2004; 50:305–313.

19. Li YH, Liu RH, Jiao LH, Wang WH. Synergetic effects of epidermal growth factor and estradiol on cytoplasmic maturation of porcine oocytes. Zygote. 2002; 10:349–354.

20. Marques MG, Nicacio AC, de Oliveira VP, Nascimento AB, Caetano HVA, Mendes CM, Mello MRB, Milazzotto MP, D'AvilaAssumpção MEO, Visintin JA. In vitro maturation of pig oocytes with different media, hormone and meiosis inhibitors. Anim Reprod Sci. 2007; 97:375–381.

21. Mattioli M, Galeati G, Bacci ML, Seren E. Follicular factors influence oocyte fertilizability by modulating the intercellular cooperation between cumulus cells and oocyte. Gamete Res. 1988; 21:223–232.

22. Moor RM, Polge C, Willadsen SM. Effect of follicular steroids on the maturation and fertilization of mammalian oocytes. J Embryol Exp Morphol. 1980; 56:319–335.

23. Ocaña Quero JM, Millán MM, Merlin MP, Mariscal MAO, Franganillo AR. In vitro bovine embryos production: influence of serum and hormonal supplementation. Arch Zootec. 1999; 48:71–74.
26. Racowsky C. Androgenic modulation of cyclic adenosine monophosphate (cAMP)-dependent meiotic arrest. Biol Reprod. 1983; 28:774–787.

27. Richter L, Romeny E, Weitze KF, Zimmermann F. Deep freezing of boar sperm. VII. Laboratory and field experiments using the Hülsenberg VIII extender. Dtsch Tierarztl Wochenschr. 1975; 82:155–162.
28. Sanbuissho A, Threlfall WR. The effects of estrous cow serum on the in vitro maturation and fertilization of the bovine follicular oocyte. Theriogenology. 1989; 31:693–699.

29. Schellander K, Fuhrer F, Brackett BG, Korb H, Schleger W. In vitro fertilization and cleavage of bovine oocytes matured in medium supplemented with estrous cow serum. Theriogenology. 1990; 33:477–485.

30. Smith DM, Tenney DY. Effects of steroids on mouse oocyte maturation in vitro. J Reprod Fertil. 1980; 60:331–338.

31. Somfai T, Kikuchi K, Onishi A, Iwamoto M, Fuchimoto D, Papp AB, Sato E, Nagai T. Relationship between the morphological changes of somatic compartment and the kinetics of nuclear and cytoplasmic maturation of oocytes during in vitro maturation of porcine follicular oocytes. Mol Reprod Dev. 2004; 68:484–491.

32. Suzuki H, Jeong BS, Yang X. Dynamic changes of cumulus-oocyte cell communication during in vitro maturation of porcine oocytes. Biol Reprod. 2000; 63:723–729.

33. Tesarik J, Mendoza C. Nongenomic effects of 17 beta-estradiol on maturing human oocytes: relationship to oocyte developmental potential. J Clin Endocrinol Metab. 1995; 80:1438–1443.

34. Tesarik J, Mendoza C. Direct non-genomic effects of follicular steroids on maturing human oocytes: oestrogen versus androgen antagonism. Hum Reprod Update. 1997; 3:95–100.

35. Wu J, Carrell DT, Wilcox AL. Development of in vitro-matured oocytes from porcine preantral follicles following intracytoplasmic sperm injection. Biol Reprod. 2001; 65:1579–1585.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download