Abstract
Vitamin D3 up-regulated protein 1 (VDUP1) is a potent growth suppressor that inhibits tumor cell proliferation and cell cycle progression when overexpressed. In a previous study, we showed that VDUP1 knockout (KO) mice exhibited accelerated liver regeneration because such animals could effectively control the expression of cell cycle regulators that drive the G1-to-S phase progression. In the present study, we further investigated the role played by VDUP1 in initial priming of liver regeneration. To accomplish this, VDUP1 KO and wild-type (WT) mice were subjected to 70% partial hepatectomy (PH) and sacrificed at different times after surgery. The hepatic levels of TNF-α and IL-6 increased after PH, but there were no significant differences between VDUP1 KO and WT mice. Nuclear factor-κB (NF-κB), c-Jun-N-terminal kinase (JNK), and signal transducer and activator of transcription 3 (STAT-3) were activated much earlier and to a greater extent in VDUP1 KO mice after PH. A single injection of TNF-α or IL-6 caused rapid activation of JNK and STAT-3 expression in both mice, but the responses were stronger and more sustained in VDUP1 KO mice. In conclusion, our findings provide evidence that VDUP1 plays a role in initiation of liver regeneration.
Vitamin D3 up-regulated protein 1 (VDUP1) is a multifunctional 46-kDa protein originally identified as differentially expressed in 1α,25-dihydroxyvitamin D3-treated HL-60 leukemia [4] and B16 melanoma cells [29]. VDUP1 expression is induced by growth arrest stimuli that block the cell cycle [16], and overexpression of VDUP1 inhibits tumor cell growth via arrest of cell cycle progression [25]. Both point mutation and knockout (KO) of VDUP1 in a mouse model were associated with a high incidence of hepatocellular carcinoma (HCC) development [17,28]. In particular, VDUP1 was shown to modulate transcription of cyclin A2 via association with co-repressor complexes, including histone deacetylase 1 [12]. Additionally, VDUP1 interacts with Jun activation domain-binding protein 1 (Jab1) and regulates the expression of several signaling genes important in cell cycle progression [15].
The liver has a remarkable regenerative potential, allowing recovery from the functional deficit after hepatic injury [8,9,23]. Experimentally, partial hepatectomy (PH) in rodents has been used extensively to explore the molecular, cellular, and physiological mechanisms that control the highly regulated response to injury. After PH, hepatocytes synchronously exit G0, re-enter the cell cycle, and undergo 1~2 rounds of replication before re-entry into quiescence [9,22]. Hepatocyte cell cycle entry during regeneration occurs in two steps. During the initial priming phase, hepatocytes transit from G0 to G1 through a highly regulated process controlled by various cytokines including tumor necrosis factor (TNF)-α and interleukin (IL)-6 [2,7,32]. These factors activate transcription factors such as nuclear factor-κB (NF-κB), c-Jun-N-terminal kinase (JNK), and signal transducer and activator of transcription 3 (STAT-3), followed by expression of other genes that encode cell cycle regulators including cyclin D [5,6]. In the second phase, growth factor stimulation triggers a G1-to-S phase transition and cell cycle progression [9,31].
We previously showed that liver regeneration after PH was significantly accelerated in VDUP1 KO mice [18]. This was attributed to enhanced cell proliferation and cell cycle progression driven, in part, via increases in signaling through the Extracellular signal-regulated kinases1/2 and Akt (Protein Kinase B) pathways. To more fully characterize the mechanisms through which VDUP1 regulates hepatocyte proliferation and liver growth, the present study was conducted to investigate the pattern of gene expression involved in the initial priming phase of liver regeneration. We found that, after PH, VDUP1 impaired timely activation of NF-κB, JNK, and STAT-3.
The VDUP1 KO mouse line used in the present study has previously been described [19]. PH of male VDUP1 KO and wild-type (WT) mice (of the C57BL/6 background) aged 8~10 weeks was conducted following the method described by Higgins and Andersen [13]. Mice were then sacrificed at various time points and liver remnants were removed, weighed, and snap-frozen in liquid nitrogen. At each time point, 3~5 mice of either genotype were sacrificed. For experiments involving TNF-α or IL-6, mice aged 5 weeks were injected intravenously (i.v.) with 25 µg/kg of recombinant murine TNF-α or IL-6 (R&D Systems, USA) dissolved in saline. Mice were sacrificed and analyzed at the time points indicated in the Results. All experiments were performed in compliance with the Guide for Care and Use of Laboratory Animals, Institute for Laboratory Animal Research (ILAR), USA.
Total RNA was extracted from liver samples using TRIzol reagent (Invitrogen, USA) according to the manufacturer's instructions. Total RNA was reversetranscribed and analyzed by real-time quantitative polymerase chain reaction (PCR) using SYBR Green PCR master mix (Applied Biosystems, USA) and targetspecific primers (Table 1). Reactions were amplified and quantified using the ABI 7700 sequence detection system and the manufacturer's software (Applied Biosystems). Target mRNA expression levels were normalized to those of β-actin, which served as an internal control.
Equal amounts of nuclear protein (40 µg) from liver extracts were separated by sodium dodecyl sulfatepolyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Antibodies against the following proteins were used as primary antibodies: NF-κB p50 (1:1,000; Assay Designs, USA), NF-κB p65 (1:1,000; Cell Signaling Technology, USA), STAT-3 (1:1,000; Cell Signaling), and phosphorylated c-jun (1:1,000; Cell Signaling). To ensure equal loading, we stained membranes with Ponceau dye (Sigma-Aldrich, USA). Immunoreactive bands were quantified by densitometry. Levels of protein expression were not normalized to levels of internal standards.
To determine the effect of VDUP1 on priming of liver regeneration, we examined the mRNA expression levels of TNF-α and IL-6. Hepatic TNF-α was evident 1 and 24 h after PH in both mice. However, no difference was observed between VDUP1 KO and WT mice (Fig. 1A). The mRNA level of IL-6 was also induced in a similar manner in VDUP1 KO mice when compared with WT mice (Fig. 1B).
TNF-α is a potent inducer of NF-κB activation in the liver. TNF-α-dependent JNK activation is involved in triggering of cell cycle progression after PH [2,32]. The p50 and p65 levels revealed that NF-κB activation was greater after PH in VDUP1 KO mice than in WT mice (Fig. 2A). The maximum NF-κB p50 subunit level in VDUP1 KO mice was observed 12 h after PH, which was earlier than in WT mice. The p65 level increased progressively to 12 h after surgery in both VDUP1 KO and WT mice, but the response was higher in VDUP1 KO mice. JNK activation (based on the level of phosphorylated c-jun) was significantly higher in VDUP1 KO mice. At 12 h, c-jun expression peaked in both mice, but the expression level was greater in VDUP1 KO mice (Fig. 2B).
We also analyzed activation of STAT-3, an IL-6-regulated kinase [6]. WT mice displayed maximal STAT-3 activation 12 h after PH, whereas in VDUP1 KO mice peak activation was observed 4 h after resection (Fig. 2C). These results indicate that VDUP1 deficiency in regenerating liver stimulates the progression of hepatocytes to the prereplicative (G1) phase via a TNF-α/IL-6-stimulated acute-phase mechanism.
We previously showed that VDUP1 regulated NF-κB via the intermediacy of TNF-α [17]. In the present study, we further explored whether VDUP1 affected JNK or STAT-3 activation mediated by TNF-α or IL-6. Treatment with TNF-α led to rapid activation of c-jun in both mice (Fig. 3A); however, the response was significantly greater in VDUP1 KO mice than WT mice. Similarly, STAT-3 activation was more pronounced in IL-6-treated VDUP1 KO mice than WT mice (Fig. 3B).
Liver regeneration is a complicated process involving multiple factors and pathways [23]. In a previous study, we showed that loss of VDUP1 accelerated liver regeneration by controlling the expression of cell cycle regulators that drive the G1-S phase progression [18]. Here, we provide several additional lines of evidence that VDUP1 plays an inhibitory role during initiation of liver regeneration.
During the first few hours after PH, various genes involved in cytokine signaling are induced to prime resting hepatocytes for cell division [4]. TNF-α and IL-6 are strongly induced in this process [4], and genetic studies in mice have confirmed that liver regeneration is impaired in animals homozygous for mutations in the genes encoding TNF-α or IL-6 [29]. Yamada et al. showed that lack of signaling through TNFR-1 greatly inhibited DNA replication after PH and caused significant mortality 24~40 h after operation [32]. IL-6-deficient mice showed high mortality after PH and DNA synthesis in hepatocytes was impaired after surgery [7]. In addition, administration of anti-TNF-α antibodies to rats inhibited liver regeneration after PH [2], whereas injection of IL-6 into IL-6 KO mice 30 min before surgery rescued the impaired response to PH [7]. To analyze the contribution of VDUP1 to the priming phase of liver regeneration, we first determined whether accelerated liver regeneration in VDUP1 KO mice was attributable to abnormal production of TNF-α and/or IL-6. However, we found no significant differences in the hepatic expression levels of TNF-α and IL-6 when VDUP1 KO and WT mice were compared.
Shortly after PH, TNF-α is rapidly secreted from Kupffer cells, inducing activation of NF-κB and JNK [2,32]. The roles played by NF-κB and JNK during liver regeneration have previously been investigated, although the details remain controversial. Absence of NF-κB activation in Kupffer cell-depleted mice impaired liver regeneration after PH [1], whereas deletion of a hepatocyte-specific inhibitor of B kinase (IKK) promoted earlier hepatocyte proliferation [21]. Conditional c-jun KO mice exhibited impaired liver regeneration [3], and inhibition of JNK delayed hepatocyte proliferation [27]. In the present study, VDUP1 KO mice showed significant increases in NF-κB and JNK activation levels after PH when compared with WT mice, suggesting that VDUP1 regulates hepatocyte proliferation via TNF-α-dependent signaling. NF-κB and JNK have been shown to regulate the expression of a number of exogenous and endogenous growth factors known to promote cellular proliferation. Multiple NF-κB binding sites have been reported in the promoter region of cyclin D1, an important cell cycle regulator that, upon association with cdk4 and cdk6, promotes the progression of cells from G0/G1 to S phase [10]. The c-jun controls liver regeneration by repressing p53/p21 expression [30], and inhibition of JNK activity in the liver suppresses cyclin D1 expression [27]. We previously showed that the cyclin D1 level was significantly increased in VDUP1 KO mice following PH, whereas that of p21 was reduced [18]. Our present results, together with those of previous studies, provide a molecular link between the increased acute phase response and the enhanced G1/S-phase transition evident in VDUP1 KO mice.
To determine whether an increase in cytokine-mediated signaling is a general feature of TNF-α-regulated responses, we compared the extent of downstream signaling from IL-6 after PH. Binding of IL-6 causes dimerization of the receptor, as well as activation of tyrosine kinases that phosphorylate gp130 and create docking sites for STAT-3 binding. Ablation of the hepatic STAT-3 gene diminished hepatocyte proliferation after PH or administration of CCl4 [11,20,24]. Overexpression of constitutively activated STAT-3 via adenoviral gene transfer attenuated liver injury and promoted hepatocyte proliferation in a rat model featuring 20% partial liver transplantation [14]. In the present study, we demonstrated that the activation of STAT-3 in VDUP1 KO mice was significantly higher than in WT mice following PH. The IL-6/STAT-3 signaling pathway is involved in cell proliferation via induction of cyclins D1, D2, D3, A, and cdc25A, and concomitant downregulation of the cyclin-dependent kinase (cdk) inhibitors p21 and p27 [26]. Thus, our results reinforce the notion that a pathway involving IL-6/STAT-3 may also contribute sequentially to increased G1 progression in VDUP1 KO mice.
Despite comparable TNF-α and IL-6 production levels after PH, VDUP1 KO mice exhibited earlier, stronger, and more sustained NF-kB, JNK, and STAT-3 activations than WT mice. In addition, a single injection of TNF-α or IL-6 into VDUP1 KO mice strongly activated JNK and STAT-3 expression. In a previous study, we showed that loss of VDUP1 increased susceptibility to chemically-induced hepatocarcinogenesis [17]. We found that an increase in cell proliferation attributable to TNF-α-induced NF-κB activation was a principal mechanism by which the absence of VDUP1 increased the extent of development of HCC. Although possible effects of VDUP1 on expression of TNF-α and IL-6 were not addressed in this study, VDUP1 was shown to be able to activate the NF-κB, JNK, and STAT-3 signal transduction pathways in a TNF-α- and IL-6-dependent manner during liver regeneration.
In conclusion, the present study investigated the role played by VDUP1 in initial priming of liver regeneration. We found that VDUP1 served as an important regulator suppressing liver regeneration after PH via modulating activation of NF-κB, JNK, and STAT-3. Accordingly, the regulation of VDUP1 may be a potential therapeutic target for the prevention and treatment of insufficient liver regeneration after segmental liver transplantation or resection of liver tumors.
Figures and Tables
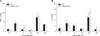 | Fig. 1Expression of genes encoding cytokines after partial hepatectomy (PH). The mRNA levels of hepatic tumor necrosis factor (TNF)-α (A) and interleukin (IL)-6 (B) were evaluated by real-time quantitative PCR, normalizing RNA relative to β-actin as the internal control. The values are the means ± SE (n = 3~4 for each group at each time point). VDUP1 KO: Vitamin D3 up-regulated protein 1 knockout. |
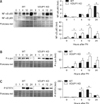 | Fig. 2Activation of signaling pathways in regenerating liver after PH. Western blotting to detect expression of the nuclear factor-κB (NF-κB) p50 and p65 subunits (A), c-jun (B), and signal transducer and activator of transcription 3 (STAT-3) (C) in hepatic nuclear extracts. Densitometric changes are expressed as percentages of the WT values at 0 h. Values are the means ± SE (n = 3~4 for each group at each time point). *p < 0.05 vs. WT mice at the respective time points. |
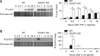 | Fig. 3The effects of TNF-α or IL-6 on c-Jun-N-terminal kinase and STAT-3 activation. Expression levels of c-jun (A) and STAT-3 (B). Nuclear proteins were extracted at the indicated times after TNF-α or IL-6 treatment, gel-separated, and immunoblotted using the indicated antibodies. Densitometric changes are expressed as percentages of the WT value at 0 h. Values are the means ± SE (n = 3~4 for each group at each time point). *p < 0.05 vs. the WT mice at the respective time points. |
References
1. Abshagen K, Eipel C, Kalff JC, Menger MD, Vollmar B. Loss of NF-κB activation in Kupffer cell-depleted mice impairs liver regeneration after partial hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2007; 292:G1570–G1577.

2. Akerman P, Cote P, Yang SQ, McClain C, Nelson S, Bagby GJ, Diehl AM. Antibodies to tumor necrosis factor-alpha inhibit liver regeneration after partial hepatectomy. Am J Physiol. 1992; 263:G579–G585.

3. Behrens A, Sibilia M, David JP, Möhle-Steinlein U, Tronche F, Schütz G, Wagner EF. Impaired postnatal hepatocyte proliferation and liver regeneration in mice lacking c-jun in the liver. EMBO J. 2002; 21:1782–1790.

4. Chen KS, DeLuca HF. Isolation and characterization of a novel cDNA from HL-60 cells treated with 1,25-dihydroxyvitamin D-3. Biochim Biophys Acta. 1994; 1219:26–32.

5. Cressman DE, Greenbaum LE, Haber BA, Taub R. Rapid activation of post-hepatectomy factor/nuclear factor κB in hepatocytes, a primary response in the regenerating liver. J Biol Chem. 1994; 269:30429–30435.

6. Cressman DE, Diamond RH, Taub R. Rapid activation of the Stat3 transcription complex in liver regeneration. Hepatology. 1995; 21:1443–1449.

7. Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996; 274:1379–1383.

8. Diehl AM. Effect of ethanol on tumor necrosis factor signaling during liver regeneration. Clin Biochem. 1999; 32:571–578.

10. Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS Jr. NF-κB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999; 19:5785–5799.

11. Haga S, Ogawa W, Inoue H, Terui K, Ogino T, Igarashi R, Takeda K, Akira S, Enosawa S, Furukawa H, Todo S, Ozaki M. Compensatory recovery of liver mass by Akt-mediated hepatocellular hypertrophy in liver-specific STAT3-deficient mice. J Hepatol. 2005; 43:799–807.

12. Han SH, Jeon JH, Ju HR, Jung U, Kim KY, Yoo HS, Lee YH, Song KS, Hwang HM, Na YS, Yang Y, Lee KN, Choi I. VDUP1 upregulated by TGF-β1 and 1,25-dihydorxyvitamin D3 inhibits tumor cell growth by blocking cell-cycle progression. Oncogene. 2003; 22:4035–4046.

13. Higgins GM, Anderson RM. Experimental pathology of the liver. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931; 12:186–202.
14. Huda KASM, Guo L, Haga S, Murata H, Ogino T, Fukai M, Yagi T, Iwagaki H, Tanaka N, Ozaki M. Ex vivo adenoviral gene transfer of constitutively activated STAT3 reduces post-transplant liver injury and promotes regeneration in a 20% rat partial liver transplant model. Transpl Int. 2006; 19:415–423.

15. Jeon JH, Lee KN, Hwang CY, Kwon KS, You KH, Choi I. Tumor suppressor VDUP1 increases p27kip1 stability by inhibiting JAB1. Cancer Res. 2005; 65:4485–4489.

16. Junn E, Han SH, Im JY, Yang Y, Cho EW, Um HD, Kim DK, Lee KW, Han PL, Rhee SG, Choi I. Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J Immunol. 2000; 164:6287–6295.

17. Kwon HJ, Won YS, Suh HW, Jeon JH, Shao Y, Yoon SR, Chung JW, Kim TD, Kim HM, Nam KH, Yoon WK, Kim DG, Kim JH, Kim YS, Kim DY, Kim HC, Choi I. Vitamin D3 upregulated protein 1 suppresses TNF-α-induced NF-κB activation in hepatocarcinogenesis. J Immunol. 2010; 185:3980–3989.

18. Kwon HJ, Won YS, Yoon YD, Yoon WK, Nam KH, Choi IP, Kim DY, Kim HC. Vitamin D3 up-regulated protein 1 deficiency accelerates liver regeneration after partial hepatectomy in mice. J Hepatol. 2011; 54:1168–1176.

19. Lee KN, Kang HS, Jeon JH, Kim EM, Yoon SR, Song H, Lyu CY, Piao ZH, Kim SU, Han YH, Song SS, Lee YH, Song KS, Kim YM, Yu DY, Choi I. VDUP1 is required for the development of natural killer cells. Immunity. 2005; 22:195–208.

20. Li W, Liang X, Kellendonk C, Poli V, Taub R. STAT3 contributes to the mitogenic response of hepatocytes during liver regeneration. J Biol Chem. 2002; 277:28411–28417.

21. Malato Y, Sander LE, Liedtke C, Al-Masaoudi M, Tacke F, Trautwein C, Beraza N. Hepatocyte-specific inhibitor-of-kappaB-kinase deletion triggers the innate immune response and promotes earlier cell proliferation during liver regeneration. Hepatology. 2008; 47:2036–2050.

24. Moh A, Iwamoto Y, Chai GX, Zhang SS, Kano A, Yang DD, Zhang W, Wang J, Jacoby JJ, Gao B, Flavell RA, Fu XY. Role of STAT3 in liver regeneration: survival, DNA synthesis, inflammatory reaction and liver mass recovery. Lab Invest. 2007; 87:1018–1028.

25. Nishiyama A, Matsui M, Iwata S, Hirota K, Masutani H, Nakamura H, Takagi Y, Sono H, Gon Y, Yodoi J. Identification of thioredoxin-binding protein-2/vitamin D3 up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J Biol Chem. 1999; 274:21645–21650.

26. Salazar-Montes A, Ruiz-Corro L, Sandoval-Rodriguez A, Lopez-Reyes A, Armendariz-Borunda J. Increased DNA binding activity of NF-κB, STAT-3, SMAD3 and AP-1 in acutely damaged liver. World J Gastroenterol. 2006; 12:5995–6001.

27. Schwabe RF, Bradham CA, Uehara T, Hatano E, Bennett BL, Schoonhoven R, Brenner DA. c-Jun-N-terminal kinase drives cyclin D1 expression and proliferation during liver regeneration. Hepatology. 2003; 37:824–832.

28. Sheth SS, Bodnar JS, Ghazalpour A, Thipphavong CK, Tsutsumi S, Tward AD, Demant P, Kodama T, Aburatani H, Lusis AJ. Hepatocellular carcinoma in Txnip-deficient mice. Oncogene. 2006; 25:3528–3536.

29. Song H, Cho D, Jeon JH, Han SH, Hur DY, Kim YS, Choi I. Vitamin D3 up-regulating protein 1 (VDUP1) antisense DNA regulates tumorigenicity and melanogenesis of murine melanoma cells via regulating the expression of fas ligand and reactive oxygen species. Immunol Lett. 2003; 86:235–247.

30. Stepniak E, Ricci R, Eferl R, Sumara G, Sumara I, Rath M, Hui L, Wagner EF. c-Jun/AP-1 controls liver regeneration by repressing p53/p21 and p38 MAPK activity. Genes Dev. 2006; 20:2306–2314.

31. Talarmin H, Rescan C, Cariou S, Glaise D, Zanninelli G, Bilodeau M, Loyer P, Guguen-Guillouzo C, Baffet G. The mitogen-activated protein kinase kinase/extracellular signal-regulated kinase cascade activation is a key signalling pathway involved in the regulation of G1 phase progression in proliferating hepatocytes. Mol Cell Biol. 1999; 19:6003–6011.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download