Abstract
We analyzed alcoholic extracts of herbs possessing anti-neosporal activity against Neospora (N.) caninum. To identify the chemical components of Sophora (S.) flavescens and Torilis (T.) japonica associated with anti-neosporal activity, specific fractions were isolated by high-performance liquid chromatography (HPLC). In vitro activity of the fractions against N. caninum was then assessed. Gas chromatography/mass spectrometry (GC/MS) was used to identify and quantify specific anti-neosporal molecules in the herbal extracts. Almost all HPLC fractions of S. flavescens and T. japonica had higher levels of anti-neosporal activity compared to the not treated control. Active constituents of the extracts were sophoridane, furosardonin A, and tetraisopropylidene-cyclobutane in S. flavescens; 5,17-β-dihydroxy-de-A-estra-5,7,9,14-tetraene, furanodiene, and 9,12-octadecadienoic acid (Z,Z)-(CAS,1) in T. japonica.
Neospora (N.) caninum is an extra-intestinal coccidian. Transplacental infection by tachyzoites is a recognized mode of neosporosis transmission [8]. A second mode of transmission involves the ingestion of tissue containing N. caninum cysts [8]. Some reports have described the anti-neosporal activities of anti-protozoal drugs including toltrazuril sulfone (Ponazuril), the calcium ionophore A23187, dihydroxyisoflavone and trihydroxydeoxybenzoin derivatives, apicidin, clindamycin followed by sulfadiazine and trimethoprim, sulfonamides; and the dihydrofolate reductase/thymidylate synthase inhibitors pyrimethamine, sulfadiazine, and amprolium [1,3,5-7,10-14,20,21,23,27,28]. We previously used an in vitro assay to measure the ability of herbal extracts to prevent N. caninum from invading Vero cells. Alcoholic extracts of Sophora (S.) flavescens and Torilis (T.) japonica were found to have high levels of anti-neosporal activity in vitro [34]. However, no active drug has been commercially produced. Thus, we sought to develop anti-neosporal drugs from S. flavescens and Torilis T. japonica. Extracts of S. flavescens and Torilis T. japonica were fractionated by high-performance liquid chromatography (HPLC), and in vitro anti-protozoal effects of the complete herbal extracts and HPLC fractions have been evaluated [33-35].
To more accurately study the active components in the extracts of S. flavescens and Torilis T. japonica, fractions were isolated with HPLC in the present study. Active compounds in the individual fractions were identified and quantified by Gas chromatography/mass spectrometry (GC/MS). In vitro effects of each fraction against N. caninum were then evaluated. For this, the fractions were subjected to a two-fold serial dilution and added to wells containing replicating N. caninum merozoites. To assess the ability of each fraction to inhibit parasite proliferation, colonies containing N. caninum tachyzoites were counted and the numbers were compared to those of an untreated control.
Anti-coccidial effects of 15 different herbal extracts were previously evaluated [33]. Extracts from S. flavescens and T. japonica were found to be more effective than extracts from other plants. Thus, extracts of these herbs were obtained with alcohol and anti-neosporal activity of the extracts was studied. Thirty g of dried S. flavescens root or T. japonica fruit were pulverized, and extracts were prepared using 200 mL of 80% (v/v) ethanol at room temperature during overnight. The extracts were lyophilized by the lyophilizer (Operon, Korea), redissolved in 10 mL of 80% (v/v) ethanol, and serially diluted from 1 : 2 to 1 : 128. Twenty µL aliquots of these solutions were added to 200 µL of RPMI 1640 medium (Difco, USA) in 96-well tissue culture plates (Corning, USA) according to the previous research [34].
For HPLC, S. flavescens and T. japonica ethanol extracts were diluted with methanol (9 : 1), and 100- or 200-µL aliquots were injected into an HPLC system consisting of an LC 250 pump (Perkin Elmer, USA), ISS 200 auto-sampler (Perkin Elmer), LC 90 UV detector (Perkin Elmer) set at 220 nm, and a strip chart recorder (Perkin Elmer). A semipreparative Microsorb C-18 column (1.2 cm × 30 cm; Rainin, USA) was used. The mobile phase consisted of an acetonitrile : water solution (3 : 1, v/v) pumped at a rate of 2 mL/min. Individual peaks or peak groups were recorded and same fractions were combined. Acetonitrile was removed by evaporation at low temperature (about 10℃) under a stream of nitrogen or air. Residual water in each fraction was removed by freeze-drying. The HPLC fractions were serially diluted from 1 : 2 to 1 : 128 and added to Dulbecco's modified Eagle's medium (DMEM) (Difco) containing Vero cells (CRL6318; ATCC, USA) in which N. caninum tachyzoites (strain KBA-2; NVRQS, Korea) were growing. Both the undiluted stock extract and diluted samples were tested according to the previous research [35].
Vero cells were plated (5 × 104 cells/well) into flat-bottomed 96-well tissue culture plates with 180 µL DMEM containing 10% (v/v) fetal bovine serum (FBS) (Gibco BRL, USA), 100 U penicillin G/mL (Gibco BRL), and 100 ng streptomycin/mL (Gibco BRL). The cells were allowed to adhere to the culture plate for 24~48 h at 37℃ in an atmosphere of 5% (v/v) CO2 and 95% air until a confluent cell monolayer was observed by inverted phase-contrast microscopy (Olympus, Japan). Next, the cultured Vero cells were inoculated with 10,000 N. caninum tachyzoites per well of culture plates. To assess the anti-protozoal effects of each HPLC fraction, the tachyzoites colonies were examined 2 days post-inoculation with an inverted microscope. The tachyzoites colonies in three microscopic fields (100×magnification) were counted 2 days post-inoculation.
N. caninum tachyzoites were harvested from the Vero cells by use of the plastic cell scraper (Guaangzhou Jet Bio-Filtration Products, China), washed in calcium- and magnesium-free Hanks' balanced salt solution (HBSS) (Gibco BRL), counted with a hemocytometer (Sigma-Aldrich, USA), and maintained in Minimum Essential Medium (MEM) (Gibco BRL) containing 10% (v/v) equine serum (Gibco BRL), 4 mM L-glutamine, MEM vitamin solution (Gibco BRL), MEM essential amino acid solution (Gibco BRL), MEM non-essential amino acid solution (Gibco BRL), antibiotic-antimycotic solution [100 U penicillin G/mL, 100 µL/mL streptomycin, and 0.25 µg/mL amphotericin B (Gibco BRL)], and 7.5% (v/v) sodium bicarbonate. Cells were allowed to adhere to culture plates for 24~48 h at 37℃ in a humidified incubator (Forma Scientific, USA) containing 5% CO2 and 95% air. When 75% of the cells were infected with tachyzoites, the cells were harvested using a plastic cell scraper (Guaangzhou Jet Bio-Filtration Products). The suspension was passed twice through a syringe (Green Cross Medical, Korea) equipped with a 23-gauge needle (Green Cross Medical) and then through a sterile filter with 5-µM pores (Gibco BRL). Tachyzoites in the filtrate were washed in cold 0.01 M PBS, collected by centrifugation (500 × g for 5 min) at room temperature, and counted with a hemocytometer (Sigma-Aldrich).
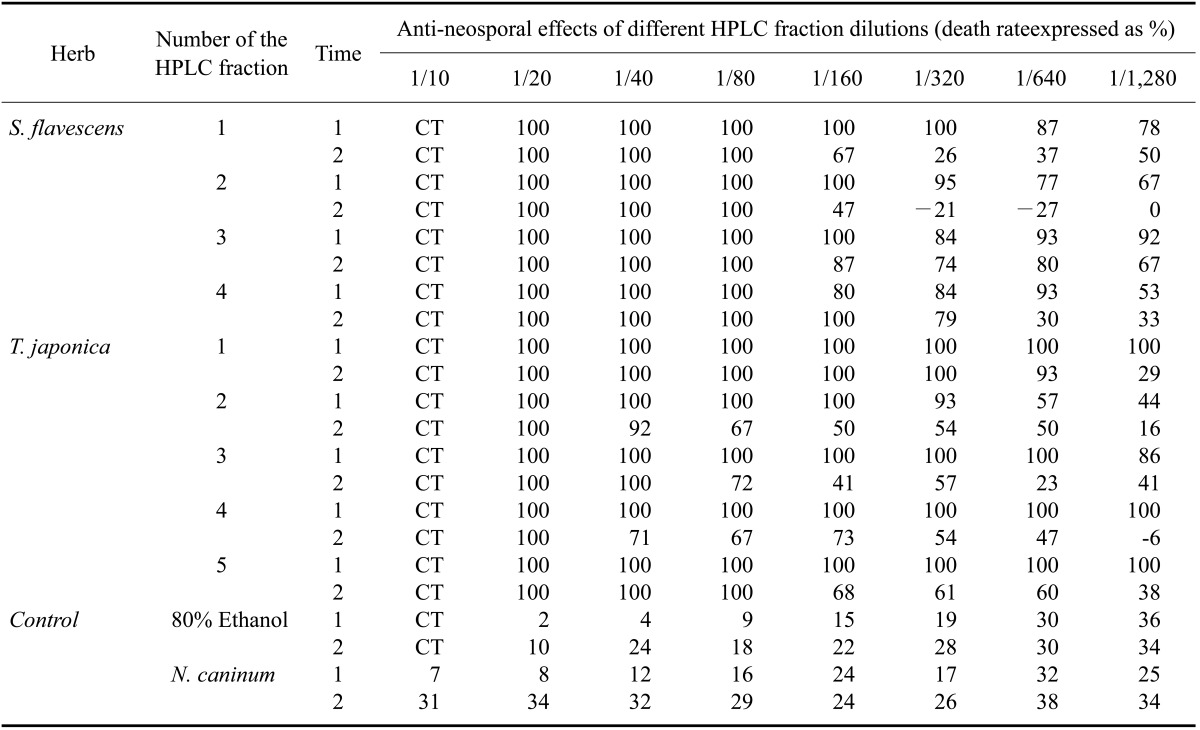
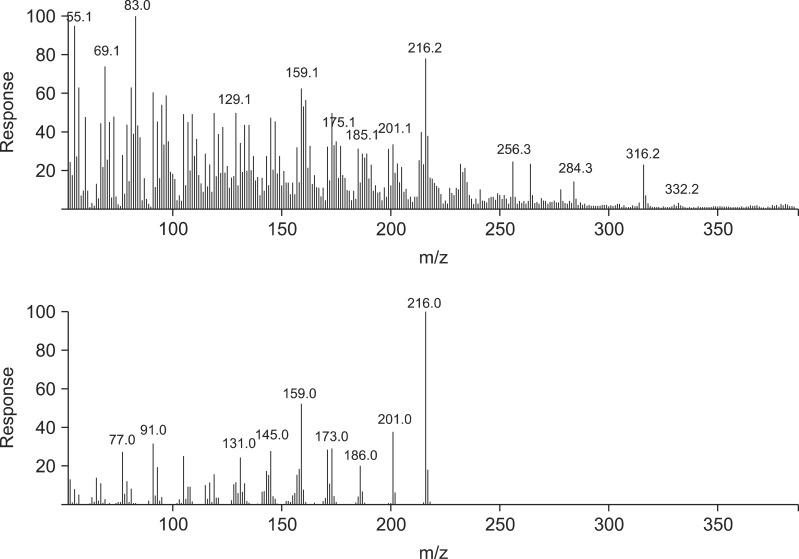
GC/MS was performed using an Agilent 6890 gas chromatograph coupled to an Agilent 5973 mass spectrometer running Agilent ChemStation software (Agilent Technologies, USA). A capillary column (30 m × 0.25 mm internal diameter; CM Scientific, USA) coated with a 0.25-µM film of 5% (v/v) phenyl methyl siloxane was used for separation. The column temperature was set at 100℃ and held for 5 min to permit injection, increased to 145℃ at a rate of 5℃/min, and held at this temperature for 25 min. Next, the temperature was allowed to rise to 200℃ at a rate of at 5℃/min, and finally to 280℃ at a rate of 20℃/min. Split injection (2-µL aliquots) was performed with a spilt ratio of 10 : 1. High-purity helium served as the carrier gas at a flow rate of 1.0 mL/min. The spectrometer was operated in the electron-impact (EI) mode with a scan range of 40~550 amu, ionization energy of 70 eV, and scan time of 0.34 sec. The inlet and ionization source temperatures were 250℃ and 280℃, respectively. We analyzed HPLC fractions of S. flavescens (Sf-1, -2, -3, and -4) and T. japonica (Tj-1, -2, -3, -4, and -5) extracts.
Using HPLC, five major T. japonica fractions were recovered. Four major S. flavescens fractions were also collected. To calculate the concentration of the fractions required to inhibit N. caninum growth, 2.85 ng dry weigh/well of the fractions were tested along with serial dilutions of each fraction. The nine fractions collected by HPLC were serially diluted from 1 : 10 to 1 : 128 in parasite culture medium. Four fractions (SF-1, -2. -3, and -4) of the S. flavescens extract and four fractions (TJ-1, -2, -4 and -5) of the T. japonica extract more efficiently inhibited N. caninum proliferation than the other fractions and control in the Vero cells (Table 1).
Further testing showed that two fractions (SF-1 and -2) of S. flavescens extract and three fractions (TJ-1, -2, and -3) of T. japonica extract more efficiently inhibited N. caninum proliferation than the other fractions and control (Table 2). It was clear that fractions SF-1 and -2 along with fractions TJ-2 and -4 inhibited N. caninum proliferation more effectively that all of the other fractions. As shown the Tables 1 and 2, all of the host cells incubated with a high concentration (2~8% v/v) of ethanol died.
Four S. flavescens fractions and five T. japonica fractions were isolated and analyzed with GC/MS (Figs. 1-6). The T. japonica and S. flavescens extracts contained sophoridane (Sf-1 and Sf-2), furosardonin A (Sf-3), tetraisopropylidene-cyclobutane (Sf-4), 5,17-β-dihydroxy-de-A-estra-5;7,9,14-tetraene (Tj-1), furanodiene (Tj-2), 9,12-octadecadienoic acid (Z,Z)-(CAS,1) (Tj-3), and materials in two unknown fractions.
A lack of chemical standards is a problem for the quality control of Chinese medicine. GC/MS permits identification of chemical compounds in the absence of standards. The concentrations of identified components can also be estimated using an analog as a standard; this is a useful approach for improving quality control [30].
N-methylcysteine and alkaloids from S. flavescens affect the motility of parasitic helminths, and exert effects on isolated frog rectus and mouse ileum [29]. A neuropharmacological mechanism is active both in parasitic helminths and host tissues [29]. Some components of S. flavescens, especially 16 flavanones, three flavanols, and four pterocarpans, have been found to exert antibacterial effects against Staphylococcus aureus, Bacillus subtilis, Staphylococcus epidermis, and Propionibacterium acne [18]. These compounds also possess anti-androgenic activities [18]. Torilin, a sesquiterpene from T. japonica, promotes the cytotoxic effects of adriamycin, vinblastine, taxol, and colchicines against multidrug-resistant cancer cells (KB-V1 and MCF7/ADR lines) [16]. This compound has been reported to strongly suppress tumorigenesis by preventing tumor invasion, and inhibits angiogenesis by decreasing tube formation by vascular endothelial cells and reducing the expression of angiogenic factors by tumor cells [16]. Furanodiene is an essential oil isolated from Eugenia unifera L. (commonly called the 'pitanga cherry') which is widely distributed in tropical and subtropical regions. The cherry-like fruit is edible and the leaves are used in folk medicine to lower blood glucose levels [22]. Furanodiene strongly inhibits the growth of Bacillus cereus [24]. Furosardonin A can be isolated from Russia sardonia, a common red mushroom. This compound is concentrated in milky juice produced by the mushroom, and confers protection from snails, insects, or other predators [2].
8-lavandulyl flavonoids, kushenol I, sophoraflavanone G, kurarinone, and kuraridine were previously isolated from S. flavescens root, and were potentiated γ-aminobutyric acid (GABA) [25, 26, 31]. Oxymatrine, sophoranol, sophoridine, and matrine were also recovered from S. flavescens [32]. Torilin, their structures were established as 11-acetoxy-8-isobutyryl-4-guaien-3-one, 11-acetoxy-8-methacrylyl-4-guaien-3-one, and 11-acetoxy-8-propionyl-4-guaien-3-one were isolated and identified from T. japonica [4,19]. In another study, 4(15)-eudesmene-1β,5α-diol and 4α,15-epoxyeudesmane-1β,6α-diol were isolated from T. japonica [17]. Adriamycin, vinblastine, taxol, and colchicine also were extracted from T. japonica [15] along with α-humulene (2.4~13.2%), bicyclogermacrene (1.9~5.4%), β-caryophyllene (1.5~4.6%), and δ-cadinene (1.0~1.9%) [9]. In the present study, the T. japonica and S. flavescens extracts were found to contain sophoridane (Sf-1 and Sf-2), furosardonin A (Sf-3), tetraisopropylidene-cyclobutane (Sf-4), 5,17-β-dihydroxy-de-A-estra-5;7,9,14-tetraene (Tj-1), furanodiene (Tj-2), and 9,12-octadecadienoicacid (Z, Z) (CAS,1; Tj-3).
Previously, HPLC fractions of medicinal herb extracts of S. flavescens and T. japonica were found to possess anti-neosporal activities [33-35]. We subsequently received a certificate of patent for HPLC fractions of medicinal herb extracts of S. flavescens and T. japonica as anti-neosporal materials from commissioner of the Korean Intellectual Property Office on August 6th, 2009.
Acknowledgments
This work was supported by a Korean Research Foundation Grant (KRF-2006-005-J02902), ARPC (Agricultural Research and Promotion Center) 20030180, and Biogreen 21 2005030104 supported by the Ministry of Rural Development Administration. We thank Dr. Myung-Haing Cho for assistance with the analysis of the HPLC fractions.
References
1. Ammann P, Waldvogel A, Breyer I, Esposito M, Müller N, Gottstein B. The role of B- and T-cell immunity in toltrazuril-treated C57BL/6 WT, µMT and nude mice experimentally infected with Neospora caninum. Parasitol Res. 2004; 93:178–187. PMID: 15290254.

2. Andina D, Bernardi MD, Del Vecchio A, Fronza G, Mellerio G, Vidari G, Vita-Finzi P. Sequiterpenes from Russula sardonia. Phytochemistry. 1980; 19:93–97.
3. Behrendt JH, Taubert A, Zahner H, Hermosilla C. Studies on synchronous egress of coccidian parasites (Neospora caninum, Toxoplasma gondii, Eimeria bovis) from bovine endothelial host cells mediated by calcium ionophore A23187. Vet Res Commun. 2008; 32:325–332. PMID: 18158611.
4. Cho WI, Choi JB, Lee K, Chung MS, Pyun YR. Antimicrobial activity of torilin isolated from Torilis japonica fruit against Bacillus subtilis. J Food Sci. 2008; 73:M37–M46. PMID: 18298734.
5. Darius AK, Mehlhorn H, Heydorn AO. Effects of toltrazuril and ponazuril on Hammondia heydorni (syn. Neospora caninum) infections in mice. Parasitol Res. 2004; 92:520–522. PMID: 14963771.
6. Darkin-Rattray SJ, Gurnett AM, Myers RW, Dulski PM, Crumley TM, Allocco JJ, Cannova C, Meinke PT, Colletti SL, Bednarek MA, Singh SB, Goetz MA, Dombrowski AW, Polishook JD, Schmatz DM. Apicidin: a novel antiprotozoal agent that inhibits parasite histone deacetylase. Proc Natl Acad Sci U S A. 1996; 93:13143–13147. PMID: 8917558.

7. Dirikolu L, Yohn R, Garrett EF, Chakkath T, Ferguson DC. Detection, quantifications and pharmacokinetics of toltrazuril sulfone (Ponazuril) in cattle. J Vet Pharmacol Ther. 2009; 32:280–288. PMID: 19646093.

8. Fuchs N, Ingold K, Sonda S, Bütikofer P, Hemphill A. Detection of surface-associated and intracellular glycoconjugates and glycoproteins in Neospora caninum tachyzoites. Int J Parasitol. 1999; 29:1597–1611. PMID: 10608447.
9. Fujita S. Miscellaneous contributions to the essential oils of plants from various territories. LI. On the components of essential oils of Torilis japonica (Houtt.) DC. Yakugaku Zasshi. 1990; 110:771–775. PMID: 2074533.
10. Gargala G, Baishanbo A, Favennec L, François A, Ballet JJ, Rossignol JF. Inhibitory activities of epidermal growth factor receptor tyrosine kinase-targeted dihydroxyisoflavone and trihydroxydeoxybenzoin derivatives on Sarcocystis neurona, Neospora caninum, and Cryptosporidium parvum development. Antimicrob Agents Chemother. 2005; 49:4628–4634. PMID: 16251305.
11. Gottstein B, Eperon S, Dai WJ, Cannas A, Hemphill A, Greif G. Efficacy of toltrazuril and ponazuril against experimental Neospora caninum infection in mice. Parasitol Res. 2001; 87:43–48. PMID: 11199848.
12. Gottstein B, Razmi GR, Ammann P, Sager H, Müller N. Toltrazuril treatment to control diaplacental Neospora caninum transmission in experimentally infected pregnant mice. Parasitology. 2005; 130:41–48. PMID: 15700756.
13. Haerdi C, Haessig M, Sager H, Greif G, Staubli D, Gottstein B. Humoral immune reaction of newborn calves congenitally infected with Neospora caninum and experimentally treated with toltrazuril. Parasitol Res. 2006; 99:534–540. PMID: 16628455.
14. Hay WH, Shell LG, Lindsay DS, Dubey JP. Diagnosis and treatment of Neospora caninum infection in a dog. J Am Vet Med Assoc. 1990; 197:87–89. PMID: 2370226.
15. Kim JH, Hwang EK, Sohn HJ, Jean YH, Yoon SS, Kim DY. Repeated bovine abortion associated with Neospora caninum in Korea. Korean J Vet Res. 1998; 38:853–858.
16. Kim SE, Kim YH, Kim YC, Lee JJ. Torilin, a sequiterpene from Torilis japonica, reverses multidrug-resistance in cancer cells. Planta Med. 1998; 64:332–334. PMID: 9619115.
17. Kitajima J, Suzuki N, Satoh M, Watanabe M. Sesquiterpenoids of Torilis japonica fruit. Phytochemistry. 2002; 59:811–815. PMID: 11937159.
18. Kuroyanagi M, Arakawa T, Hirayama Y, Hayashi T. Antibacterial and antiandrogen flavonoids from Sophora flavescens. J Nat Prod. 1999; 62:1595–1599. PMID: 10654410.
19. Lee IK, Lee JH, Hwang EI, Yun BS. New guaiane sesquiterpenes from the fruits of Torilis japonica. Chem Pharm Bull. 2008; 56:1483–1485. PMID: 18827397.
20. Lindsay DS, Butler JM, Rippey NS, Blagburn BL. Demonstration of synergistic effects of sulfonamides and dihydrofolate reductase/thymidylate synthase inhibitors against Neospora caninum tachyzoites in cultured cells, and characterization of mutants resistant to pyrimethamine. Am J Vet Res. 1996; 57:68–72. PMID: 8720241.
21. Lindsay DS, Dubey JP. Effects of sulfadiazine and amprolium on Neospora caninum (Protozoa: Apicomplexa) infections in mice. J Parasitol. 1990; 76:177–179. PMID: 2319416.
22. Matsumura T, Kasai M, Hayashi T, Arisawa M, Momose Y, Arai I, Amagaya S, Komatsu Y. α-Glucosidase inhibitors from Paraguayan natural medicine, Ñangapiry, the leaves of Eugenia uniflora. Pharm Biol. 2000; 38:302–307. PMID: 21214481.
23. Mitchell SM, Zajac AM, Davis WL, Kennedy TJ, Lindsay DS. The effects of ponazuril on development of apicomplexans in vitro. J Eukaryot Microbiol. 2005; 52:231–235. PMID: 15926999.

24. Ogunwande IA, Olawore NO, Ekundayo O, Walker TM, Schmidt JM, Setzer WN. Studies on essential oils composition, antibacterial and antitoxicity of Eugenia unifera L. Int J Aromatherapy. 2005; 15:147–152.
25. Park SJ, Nam KW, Lee HJ, Cho EY, Koo U, Mar W. Neuroprotective effects of an alkaloid-free ethyl acetate extract from the root of Sophora flavescens Ait. against focal cerebral ischemia in rats. Phytomedicine. 2009; 16:1042–1051. PMID: 19427179.
26. Piao XL, Piao XS, Kim SW, Park JH, Kim HY, Cai SQ. Identification and characterization of antioxidants from Sophora flavescens. Biol Pharm Bull. 2006; 29:1911–1915. PMID: 16946508.
27. Strohbusch M, Müller N, Hemphill A, Greif G, Gottstein B. NcGRA2 as a molecular target to assess the parasiticidal activity of toltrazuril against Neospora caninum. Parasitology. 2008; 135:1065–1073. PMID: 18549517.
28. Strohbusch M, Müller N, Hemphill A, Krebber R, Greif G, Gottstein B. Toltrazuril treatment of congenitally acquired Neospora caninum infection in newborn mice. Parasitol Res. 2009; 104:1335–1343. PMID: 19205743.
29. Terada M, Sano M, Ishii AI, Kino H, Fukushima S, Noro T. Studies on chemotherapy of parasitic helminths (IV). Effects of alkaloids from Sophora flavescens on the motility of parasitic helminths and isolated host tissues (author's transl). Nihon Yakurigaku Zasshi. 1982; 79:105–111. (Japanese). PMID: 7200047.
30. Yang FQ, Li SP, Chen Y, Lao SC, Wang YT, Dong TTX, Tsim KWK. Identification and quantitation of eleven sesquiterpenes in three species of Curcuma rhizomes by pressurized liquid extraction and gas chromatography-mass spectrometry. J Pharm Biomed Anal. 2005; 39:552–558. PMID: 15946818.
31. Yang X, Baburin I, Plitzko I, Hering S, Hamburger M. HPLC-based activity profiling for GABAA receptor modulators from the traditional Chinese herbal drug Kushen (Sophora flavescens root). Mol Divers. 2011; 15:361–372. PMID: 21207144.
32. Ye G, Zhu HY, Li ZX, Ma CH, Fan MS, Sun ZL, Huang CG. LC-MS characterization of efficacy substances in serum of experimental animals treated with Sophora flavescens extracts. Biomed Chromatogr. 2007; 21:655–660. PMID: 17370298.
33. Youn HJ, Noh JW. Screening of the anticoccidial effects of herb extracts against Eimeria tenella. Vet Parasitol. 2001; 96:257–263. PMID: 11267752.
34. Youn HJ, Lakritz J, Kim DY, Rottinghaus GE, Marsh AE. Anti-protozoal efficacy of medicinal herb extracts against Toxoplasma gondii and Neospora caninum. Vet Parasitol. 2003; 116:7–14. PMID: 14519322.
35. Youn HJ, Lakritz J, Rottinghaus GE, Seo HS, Kim DY, Cho MH, Marsh AE. Anti-protozoa efficacy of high performance liquid chromatography fractions of Torilis japonica and Sophora flavescens extracts on Neospora caninum and Toxoplasma gondii. Vet Parasitol. 2004; 125:409–414. PMID: 15482896.
Table 1
Effect of different dilutions of HPLC fractions from Sophora (S.) flavescens and Torilis (T.) japonica on the 10,000 tachyzoites/well of Neospora (N.) caninum




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download