Introduction
The somatic cell nuclear transfer (SCNT) technique has several serious problems that must be resolved to improve its efficiency [7]. These problems are considered to be a result of abnormal reprogramming of SCNT embryos [14,15]. The reprogramming of SCNT embryos can be influenced by a number of factors involving nucleo-cytoplasmic interactions [4]. Mechanical and physiochemical treatments such as enucleation, electrofusion and activation can also cause cellular stresses in the SCNT embryos. We recently reported that cellular stress that can be caused by the manipulations during SCNT procedures, resulting in generation of reactive oxygen species (ROS) in bovine SCNT embryos [13].
High levels of ROS in the SCNT embryos might result in serious damage to cell membranes [1] and nuclear and mitochondrial DNA of the cells [9, 20], including apoptosis [29], DNA fragmentation [16], abnormal metabolism [19] and aberrant gene expression [20]. Such effects can restrict reprogramming of SCNT embryos. Therefore, the present study was conducted to examine the ROS generation level and subsequent damage to mitochondrial DNA in bovine SCNT embryos.
Materials and Methods
In vitro maturation of oocytes
Bovine cumulus-oocyte complexes (COCs) were collected from the follicles (2- to 7-mm diameter) of ovaries. About ten COCs were cultured in 50 µL droplets of in vitro maturation (IVM) medium overlaid with paraffin oil for 20~22 h at 39℃ under 5% CO2 in air. The IVM medium was Tissue Culture Medium 199 (TCM199; Gibco BRL, USA) supplemented with 10% fetal bovine serum (FBS; Gibco BRL), 0.02 U/mL follicle-stimulating hormone (Sigma-Aldrich, USA), 1 µg/mL estradiol (Sigma-Aldrich), 50 µg/mL gentamicin (Sigma-Aldrich), and 0.2 mM Na-pyruvate (Sigma-Aldrich).
Preparation of donor cells
Bovine ear skin fibroblasts (4~6 passages) from a Korean native cow were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco BRL) supplemented with 10% FBS, 0.2 mM Na-pyruvate (Sigma-Aldrich), and 1% penicillin/streptomycin for 2~3 days to achieve about 70% confluency. The cells were then cultured for an additional 5 days in DMEM containing 0.5% FBS. Prior to use, the cells were trypsinized and washed by centrifugation in TCM199 medium supplemented with 3 mg/mL bovine serum albumin (BSA; Sigma-Aldrich).
Nuclear transfer
SCNT was carried out in Hepes-buffered TCM199 (Gibco BRL) supplemented with 3 mg/mL BSA and 5 µg/mL cytochalasin B (Sigma-Aldrich) according to the methods reported previously [17]. The cumulus cells of in vitro matured COCs were removed by vortexing for 5 min in phosphate-buffered saline (PBS) supplemented with 0.1% (w/v) hyaluronidase (Sigma-Aldrich) and 0.1% (w/v) polyvinyl alcohol (PVA; Sigma-Aldrich). Prior to the enucleation, oocytes were cultured in TCM199 containing 0.4 µg/mL demecolcine (Sigma-Aldrich) for 40 min to extrude their metaphase II (MII) chromosome mass. Enucleation of oocytes was undertaken by removing the MII chromosome mass and the 1st polar body. Donor cells were then injected into the perivitelline space of enucleated oocyte recipients.
Electrofusion and activation
Reconstructed oocytes were manually aligned between two wire electrodes (1-mm apart) of a fusion chamber overlaid with 0.3 M mannitol solution containing 0.1 mM MgSO4, 0.05 mM CaCl2, and 0.1% BSA. A single direct-current pulse of 1.3 kV/cm was applied for 30 µsec using a BTX Electro Cell Manipulator 200 (BTX, USA). After fusion treatment, the reconstituted oocytes were placed in CR1aa [21] containing 3 mg/mL BSA and checked for fusion. The fused oocytes were then activated using 10 µM Ca-ionophore (A23187; Sigma-Aldrich) for 5 min and cultured in CR1aa containing 3 mg/mL BSA and 2 mM 6-dimethylaminopurine (DMAP; Sigma-Aldrich) for 3 h.
In vitro fertilization (IVF)
Bovine COCs matured in vitro for 22 h were coincubated with frozen-thawed spermatozoa (2 × 106 spermatozoa/mL) in a 50 µL drop of BO medium [3] supplemented with 5 mM caffeine (Sigma-Aldrich), 10 µg/mL heparin (Sigma-Aldrich), and 3 mg/mL BSA at 39℃ under 5% CO2 in air for 6 h.
In vitro culture of embryos
After activation or insemination culture, the SCNT and IVF embryos were further cultured in a 50 µL drop of CR1aa containing 3 mg/mL BSA and 50 µg/mL gentamicin at 39℃ and 5% CO2 in air prior to analysis of ROS levels or cellular damage (18~20 h post fusion or insemination).
Analysis of ROS products
The ROS levels of SCNT and IVF embryos were measured as previously described [13]. Briefly, SCNT and IVF embryos at the zygote stage were stained with 10 µM dichlorodihydrofluorescein diacetate (H2DCFDA; Molecular Probes, USA) or 10 µM 3'-(p-hydroxyphenyl) fluorescein (HPF, Molecular Probes) for 30 min at 39℃ to determine the H2O2 level [11] or the ˙OH radical level, respectively [23]. After washing in PBS, the embryos were mounted onto glass slides and examined under a fluorescent microscope (BX-50; Olympus, Japan) with filters at 450~480 nm for excitation and 515 nm for emission. The fluorescent images from the samples were recorded as JPEG files using a digital camera (ESO 600D; Canon, Japan) and analyzed using ImageJ software 1.37 (NIH) based on the intensity of fluorescence in each embryo.
Laser-scanning confocal microscopic visualization and determination of mitochondrial membrane potential (ΔΨ)
To assess the morphology and distribution patterns of the mitochondria, SCNT and IVF embryos were stained with MitoTracker Red CMXRos dye (Molecular Probes; Invitrogen, USA) at 18~20 h after electrofusion or insemination for 30 min at 37℃. The fluorescent images of equatorial sections were obtained using a laser-scanning confocal microscope (LSM510; Zeiss, Germany) with excitation at 543 nm and emission at 560 nm.
To assess the ΔΨ, SCNT and IVF embryos were stained with 20 µg/mL 5,5',6,6'-tetrachloro-1,1,3,3'-tetraethylbenzimdazoylcarbocyanine iodide (JC-1) (Molecular Probes) at 18~20 h after electrofusion or insemination for 30 min at 37℃. The embryos were then mounted in Hepesbuffered TCM199 and visualized using a laser-scanning confocal microscope (LSM510; Zeiss) with excitation at 488 nm and emission at 500 ~ 550 nm (green) and 560 nm (red). The images of individual embryos were captured and the ratio of red to green fluorescence intensity was analyzed using the LSM Image Browser (ver. 4.20; Zeiss).
Assessment of apoptosis
Two analyzing methods were used to assess the apoptosis of SCNT embryos. A TUNEL assay was used to determine the apoptosis of SCNT and IVF embryos. The one-cell stage embryos at 18~20 h after electrofusion or insemination were fixed for 1 h in PBS (Gibco BRL) containing 4% (w/v) paraformaldehyde (Sigma-Aldrich) and then permeabilized for 30 min in PBS containing 0.5% Triton X-100 and 0.3% (w/v) polyvinylpyrrolidone (PVP) at 37℃. The fixed embryos were then incubated in TUNEL reaction medium (In Situ Cell Death Detection Kit, TMR red; Hoffmann-La Roche, Germany) in the dark for 1 h at 37℃. The embryos were then repermeabilized for 5 min in PBS containing 0.5% Triton X-100 and 0.3% (w/v) PVP at room temperature, after which they were stained for 30 min with 10 µg/mL of Hoechst 33342 (Sigma-Aldrich) at 37℃ and mounted on a glass slide in Vecta-Shield anti-fade (Vector Laboratories, USA) under a cover slip. The apoptosis of embryos was determined by fluorescence microscopy.
A caspase-3 activity assay was performed using a Caspase-3 Colorimetric Activity Assay Kit (EMD Millipore, USA). Briefly, 20 individual one-cell stage SCNT and IVF embryos were put into 1-mL tubes containing 50 µL cell lysis buffer (1×) and lysed for 15 min on ice at 18 ~ 20 h after electrofusion or insemination. Cell lysates were centrifuged for 5 min at 7,000 × g, after which supernatants were transferred into 100 µL Assay Mixture containing 10 µL Caspase-3 substrate prepared in a 96-well plate and incubated for 1 h at 37℃. The enzyme reactions were then colorimetrically monitored at 405 nm with a microtiter plate reader (Power Wave XS; BioTek, USA) by reading the OD values using the Gene 5 software.
Comet assay
The DNA damage in SCNT and IVF embryos was analyzed by comet assay at 18 ~ 20 h after electrofusion or insemination. A comet assay was performed using an OxiSelect Comet Assay Kit (Cell Biolabs, USA). Prior to assay, OxiSelect comet agarose (Assay kit no. 235002; Cell Biolabs) was melted at 90℃ for 20 min and then cooled at 37℃ for 20 min. About 75 µL of agarose was then dropped onto an OxiSelect 3-well comet slide (Assay kit no. 235001; Cell Biolabs), after which approximately ten zona-free embryos were transferred to agarose drops and chilled at 4℃ for 20 min. The samples were then lysed in lysis buffer [250 mM NaCl, 20% EDTA solution (Assay kit no. 235004; Cell Biolabs), 10% DMSO, 10% 10× lysis solution (Assay kit no. 235005; Cell Biolabs), pH ~10.0] at 4℃ for 3 h. Next, the slides were carefully transferred to chilled alkaline solution (300 mM NaOH, 1 mM EDTA) and immersed for 30 min at 4℃. Subsequently, the slides were transferred to a horizontal electrophoresis chamber (Mupid α; Advance Electric, Japan) filled with cold TAE buffer (ELPIS Biotech, Korea) and electrophoresed for 20 min at 50 V, after which the slides were stained with 1× Vista Green DNA Dye (Assay kit no. 235003; Cell Biolabs) for 20 min and then examined using a fluorescent microscope with a FITC filter. All steps after agarose treatment were conducted in the dark to prevent additional DNA damage. The comet tail lengths were measured in individual embryos using CASP (ver. 1.2.2; Zbigniew Koza, Poland).
Results
ROS levels of SCNT and IVF embryos
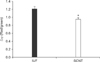
The H2O2 and ˙OH radical levels of SCNT embryos were significantly higher (p < 0.05) (35.6 ± 1.1 and 44.6 ± 1.2 pixels/embryo, respectively) than those of the IVF embryos (19.2 ± 1.5 and 23.8 ± 1.8 pixels/embryo, respectively, Fig. 1).
Mitochondrial morphology and membrane potential (ΔΨ)
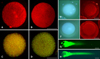
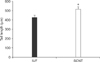
The mitochondrial morphology of IVF embryos exhibited a granular or punctate pattern, which is typical of normalmitochondria (Fig. 2A). Conversely, the mitochondrial morphology of SCNT embryos was characterized by a diffuse and non-granularity cytoplasmic staining pattern (Fig. 2B). IVF embryos showed intense red JC-1 staining with active ΔΨ, whereas SCNT embryos showed weak red JC-1 staining with reduced or inhibited ΔΨ (Fig. 2C and D). Quantitative analysis revealed that mitochondria ΔΨ (red/green) was significantly lower (p < 0.05) in SCNT embryos (0.95 ± 0.04) than IVF embryos (1.21 ± 0.06, Fig. 3).
Apoptosis
TUNEL Assay (Fig. 2E and F) revealed that no apoptosis had occurred in SCNT embryos or IVF embryos at the one-cell stage (a total of 50~55 embryos in each group, data not shown). Caspase-3 activity assay also confirmed the results of the TUNEL assay. There was no difference in the OD values of SCNT (OD 0.056, n = 101) and IVF (OD 0.057, n = 107) embryos, which were similar to that of the standard blank (OD 0.055).
DNA fragmentation
Comet assay results revealed that both IVF and SCNT embryos exhibited a clearly defined comet tail (Fig. 2G and H). However, the length of migrated DNA fragments in SCNT embryos (515.5 ± 26.4 µm) was found to be significantly (p < 0.05) longer than that of IVF embryos (425.6 ± 25.0 µm, Fig. 4).
Discussion
The results of our previous study suggested that cellular stress might occur through micromanipulation, resulting in generation of a high level of ROS production [13]. In the present study, the ROS level of SCNT embryos at the zygote stage was higher than that of IVF embryos, which confirmed the results of the previous study, in which micromanipulation procedures were found to produce ROS in the cytoplasm of SCNT embryos [13]. In the present study, we focused on evaluation of cellular damage that may be induced by excessive ROS generated during the SCNT procedure; thus, we did not evaluate the development and ROS level in later stage embryos.
ROS induces morphological and metabolic damage to mitochondria, indicative of mitochondrial dysfunction, followed by apoptotic degeneration [28]. Lipid peroxides that are induced by ROS affect vital mitochondrial functions, such as respiration and oxidative phosphorylation, inner membrane barrier properties, and maintenance of ΔΨ [2,30]. It has also been suggested that ROS activates mitochondrial permeability transition (mPT) pores that allow the release of proapoptotic factors such as mitochondrial cytochrome C [8]. In this study, the mitochondrial morphology of SCNT embryos was characterized by a diffuse and non-granular cytoplasmic staining pattern. Unlike the granular pattern of intact mitochondria, the diffuse and non-granular pattern was interpreted to indicate the release of mitochondrial CMXRos back into the cytoplasm and as evidence of an mPT [8]. In this study, ΔΨ of SCNT embryos was lower relative to IVF embryos, which is also suggestive of an mPT [28]. It has been reported that the metabolic consequence of sustained mPT is a phenomenon of mitochondrial uncoupling [27]. Uncoupled mitochondria are characterized by decreased inner mitochondrial membrane integrity and proton gradient, and subsequent reduced or abolished ATP synthesis [28].
It has been reported that higher levels of ˙OH radical derived from H2O2 induce lipid peroxidation [24] and DNA damage [9]. DNA damage induced by ROS is involved in apoptosis [29] and DNA fragmentation [12,16], which can lead to abnormal gene expression as well as suppression of further embryonic development [16]. Although no apoptosis was detected in SCNT embryos at the one-cell stage by TUNEL and caspase-3 assays in this study, apoptotic nuclei might increase during in vitro development of the SCNT embryos when compared with IVF embryos. In a previous study, apoptotic nuclei were detected from the four-cell stage and increased greatly during further in vitro culture of SCNT bovine embryos when compared to those of IVF embryos [6]. In another study, no TUNEL-positive nuclei were observed in IVF or SCNT porcine early embryos prior to day 5 of in vitro culture; however, the apoptotic rate of porcine SCNT embryos increased with culture time from day 5 to 8 and was significantly higher than in the IVF embryos [10].
The ˙OH radical assaults the deoxyribose moiety of DNA to trigger strand breaks [22,26], which induces mispairing during DNA polymerization [18] and irreversible double-strand blocks at the time of DNA replication [5]. DNA fragmentation can be detected by comet assay [25], which has been widely accepted as a sensitive tool for assessing DNA damage in animal cells. In the present study, although no apoptosis was detected by TUNEL and Caspase-3 assays, comet tail movement was observed in both SCNT and IVF one-cell embryos. The comet tail length of SCNT embryos was found to be significantly longer than that of IVF embryos, indicating that the DNA fragmentation of SCNT embryos is induced with high frequency. Such DNA fragmentation in the zygote stage SCNT embryos would result in further increases in irreversible double-strand breaks of DNA through DNA replications during embryo development [5].
In conclusion, the results of the present study show that cellular stress during micromanipulation procedures can generate ROS and subsequently induce mitochondrial and DNA damage in bovine SCNT embryos, which could lead to abnormal nuclear reprogramming of SCNT embryos.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download