Enteric diseases sporadically occur in commercial poultry worldwide and are significant economic problems because they result in decreased feed absorption, depressed weight gain, decreased flock uniformity, and increased mortality [3,6]. The causes of enteric diseases are usually complicated by the presence of other pathogens as well as nutrition, management, immune status of the affected birds, and environmental factors including suboptimal temperature [3,10,13]. The severity of enteric diseases thus ranges from subclinical to severe, especially among turkeys and broiler chickens. A major enteric disease in broiler chickens called runting stunting syndrome (RSS) is thought to be caused by a number of viruses including chicken astrovirus (CAstV), avian rotavirus (ARV), and avian reovirus [7]. Recent reports suggest that concomitant enteric viral infections may increase the severity of clinical signs in turkeys [8,11]. However, the prevalence of viral enteric diseases, especially ones caused by CAstV and ARV in broilers, has not been reported in Korea.
From May 2010 to June 2011, 21 broiler flocks including birds 1 to 4 weeks old with enteritis were submitted to the Avian Disease Laboratory of Chungbuk National University (Korea). Reverse transcription (RT)-PCR was performed to identify cases of CAstV and ARV infection using RNA extracted from pooled samples of intestinal tissues collected from each broiler flock [2]. CAstV and ARV were detected in 23.8% (5/21) and 14.3% (3/21) of the samples, respectively. During this survey, we observed an unusual type of RSS in young broilers featuring a relatively high mortality rate and severe gross intestinal lesions. We also identified concomitant infection with CAstV and ARV in these chickens. The pathological and biological characteristics of these animals were assessed.
A broiler farm located in Chungbuk, Korea contained a single flock of approximately 35,400 birds. The total number of dead and culled birds between 2 and 6 days old was approximately 3,200 (9.03%). Seven chickens aged 8 days old were submitted for necropsy. For routine bacterial isolation, liver samples were plated onto blood agar (Hanil Komed, Korea) and MacConkey agar plates (BBL, USA) and subsequently incubated at 37℃ for 24 hours. To identify the presence of Salmonella in the intestines, pooled intestinal tissues were enriched for 24 h at 41℃ in Rappaport-Vassiliadis (RV) broth (Difco, USA) followed by sub-culturing on Rambach agar (Merck, Germany) and/or MacConkey agar at 37℃ for 24 hours. Tissue samples for histopathology were fixed in 10% neutral-buffered formalin solution, embedded in paraffin, cut into sections approximately 5-µm thick, and stained with hematoxylin and eosin (H&E).
For ARV characterization, the VP6 gene of ARV was amplified using RT-PCR as previously described [4]. Sequences of the CAstV ORF1B (about 362 bp) and ARV VP6 (about 1167 bp) genes were obtained by direct sequencing using an ABI3730XL DNA analyzer (Applied Biosystems, USA). Phylogenetic trees were constructed using Molecular Evolutionary Genetics Analysis (MEGA, version 5.01) software. Nucleotide sequences of CAstV and ARV obtained in this study are available in the International Nucleotide Sequence Database under accession numbers JN635502 and JN635503, respectively.
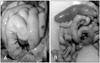
Gross lesions were commonly found in the intestines, which were thin and pale with frothy contents (Fig. 1). No other gross lesions were observed except a pale kidney with urate deposition in one chicken. Bacteria were not isolated from livers and intestines. Contrary to the obvious gross lesions, there were minimal microscopic lesions in the intestines such as mild lymphocytic infiltration in the mucosa. These findings are consistent with ones from reports that described enteritis simultaneously occurring with astroviral infection [1,5]. Evidence of astrovirus contrasts with the obvious pathological findings associated with rotaviral infection such as microscopic intestinal lesions accompanied by severe villous atrophy and crypt hyperplasia [1,5,6].
Clinical signs and gross lesions observed in the present study were significantly more severe compared to those of RSS cases previously reported that did not have a bacterial infection [3]. Differences in clinical signs and gross lesions from the present case compared to those of previous investigations may be due to different factors. One factor that influenced the present case was a concomitant infection with ARV. A recent report noted that concomitant infections with different enteric viruses induce more severe clinical signs [11]. Thus, concomitant infection with CAstV and group A ARV may have had a detrimental synergistic effect on the intestines evaluated in the current study. Another influencing factor may have been environmental temperature. The temperature on the day of the outbreak at the location of the farm was lower than that of the previous day by about 12℃ according to the Korea Meteorological Administration. It has been shown that RSS infection causes more severe enteric lesions in broilers subjected to stressful environments such as ones with suboptimal temperatures [10].
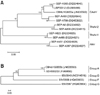
The expected amplicons for genes of CAstV ORF1B and ARV VP6 except for the reovirus S4 gene were successfully obtained [2,9]. Phylogenetic trees based on the nucleotide sequences of CAstV polymerase ORF1B and ARV VP6 showed that CAstV and ARV identified in this study were classified as CAstV and group A ARV, respectively (Fig. 2). The ARV shared a high nucleotide sequence homology (94~96%) with group A ARVs. In contrast, the CAstV identified in this study exhibited a relatively low nucleotide sequence homology (85~88%) compared to sequences of previously described CAstV strains according to a NCBI BLAST search (www.ncbi.nlm.gov/BLAST). Therefore, the CAstV detected in this study was classified as having a distinct genotype. Because the pathogenicity of astroviruses varies depending on the strain [12], the pathogenicity of the CAstV identified in the current investigation should be evaluated.
Taken together, results from the present study indicate that the intestinal lesions observed were caused by CAstV and group A ARV infection. This was the main reason for increased mortality and growth retardation of the broilers. The current report is the first to describe simultaneous CAstV and group A ARV infection of broiler chickens in Korea.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download