Introduction
Atopic dermatitis (AD) is a common chronic inflammatory skin disease that affects about 10% of the canine population in worldwide [8]. Although it is known that genetic, immunological, and environmental factors play an important role in the pathogenesis of this disorder [3], this process is not well understood. A characteristic feature of AD is impairment of the epidermal skin barrier, which may increase transepidermal water loss (TEWL) and reduce skin hydration [10]. However, most of previous studies on canine atopic dermatitis (CAD) have focused on abnormal immunological responses rather than the barrier function of the skin.
Defects in the epidermal permeability barrier function are generally viewed as a consequence of inflammation (i.e., the so-called "inside-outside" hypothesis). Alternatively, it has been predicted [13,14] that epidermal barrier dysfunction could drive AD development and progression (i.e., the "outside-inside" hypothesis). A recent study has shown that ceramide deficiency may be responsible for the lipid barrier defect occurring in dogs with CAD [23]. Ceramides are the most important class of lipids in the stratum corneum and include numerous molecular species. Canine SC has a ceramide profile close to the one reported for human SC [22].
Recent study has shown that topical mixtures of three main SC components (ceramide, cholesterol, and free fatty acids) accelerate skin barrier repair [20]. Many topical products containing physiologic lipids have been produced and are already used in human medicine [2,16,19]. However, there is lack of both products and clinical data proving the efficacy of these types of products for treating AD in veterinary medicine.
The present study was designed to evaluate the effects of a moisturizer containing physiologic lipid granules for treating CAD. A moisturizer with lipid granules composed of ceramide, cholesterol, and fatty acids was prepared. Since the granules had multiple lamellar structures, they were expected to have superior skin affinity and therefore provide high levels of moisturization.
Materials and Methods
Study population
A total of 20 dogs in Korea diagnosed with CAD based on history, typical clinical signs, positive results of serum allergen-specific IgE and/or intradermal skin tests were enrolled in this study (Table 1). Animals with ectoparasitosis and skin infections were excluded. Clinical diagnostic criteria used in the study were proposed by Willemse [24] and modified by Prelaud et al. [4]. Written informed consent for the investigation was obtained from the owners of the dogs. A food elimination trial was performed using a commercial hydrolyzed hypoallergenic diet (Royal Canin, France) for at least 8 weeks to rule out adverse food reactions as the cause of CAD. All participants had been fed a standard adult diet (Royal Canin Mini Indoor Adult Dog Food; Royal Canin, France) for at least 3 months prior to the study.
Moisturizer
A moisturizing cream containing physiologic lipid granules (Atobarrier Cream; Amorepacific, Korea) was prepared for this study. This moisturizer contained ceramide, cholesterol, and fatty acids in a ratio (3 : 1 : 1) optimal for restoring the skin barrier. In addition, panduratin, ursolic acid, niaciamide, extract of Opuntia coccinellifera, allantoin, sodium hyaluronate, and borage oil were included. This product was originally designed for human use; therefore, we adjusted the pH of this product so that it was appropriate for canine skin (pH 7.0).
Moisturizer application and evaluation procedure
The moisturizer was applied to the skin of dogs with CAD once daily for 4 weeks over the entire body except for the feet and face. The owners were also instructed to bathe the dogs once a week with hypoallergenic shampoo (Dermallay Shampoo; DermaPet, USA). The dogs were examined on day 0 of the study as well as days 14 and 28. TEWL and skin hydration were assessed. Scores for the pruritus index for canine atopic dermatitis (PICAD) and modified canine atopic dermatitis extent and severity index (CADESI) were also obtained. On day 0 and one day after the final moisturizer application, skin punch biopsy samples (10 mm) for transmission electron microscopy (TEM) evaluation were taken from the lateral thorax of five dogs under local anesthesia with 2% lidocaine hydrochloride (Lidocaine hypochloride 2%; Daihan Pharm, Korea). When necessary, three dogs were also sedated with an intravenous injection of propofol (Provive inj. 10%; Myungmoon Pharm, Korea).
Determination of skin lesion scores
The extent and severity of cutaneous lesions were assessed according to modified CADESI-03 scores [17].
Assessment of pruritus
Severity of pruritus was measured with the PICAD. The entire body was divided into head, neck, trunk (axillae), ventral abdominal, front leg, hind leg, inguinal, and ano-genital regions. Pruritus was then graded according to intensity and frequency in these eight areas [1].
Measurement of TEWL
Prior to measuring TEWL and skin hydration, the dogs were not permitted to exercise and allowed to acclimate to test room conditions. TEWL was measured using an unventilated closed chamber device (Vapometer SWL-3; Delfin Technologies, Finland) with a standard adaptor and 11-mm aperture according to the manufacturer's instructions. To standardize the measurements, hair on the lateral thorax of all the dogs was clipped. Measurements were taken in a constant environment (ambient temperature of 20~21℃ and 40~48% relative humidity) to minimize seasonal variation, and between 6 pm and 7 pm to exclude diurnal variation. On the day of examination, the dogs were not washed and nothing was applied on the skin surface [21]. All values are expressed as the median of five measurements to avoid inaccuracies.
Evaluation of skin hydration
Skin hydration of all 20 dogs was assessed by measuring skin capacitance with a Corneometer (Corneometer CM825; Courage + Khazaka Electronic GmbH, Germany) over the lateral thorax.
TEM
Skin specimens from the five dog were fixed for 8 h with 2.5% glutaraldehyde in PBS (pH = 7.0) at 4℃. Next, sections 100-µm thick were prepared with a vibratome (model G; Vibratome Oxford, USA) [15]. The sections were fixed in the 2.5% glutaraldehyde solution for 60 min at 4℃, washed twice in PBS, and post-fixed with 0.25% ruthenium tetroxide (Ruthenium tetroxide 0.5%; Electron Microscopy Science, US) in PBS (pH = 7.0) in a dark chamber at 4℃ for 60 min. After rinsing with PBS, the sections were dehydrated in a graded ethanol solution, and embedded in Spurr's resin (Spurr's resin; Polysciences, US). Ultrathin sections (70 nm) were collected on copper grids (Electron Microscopy Science, US) and stained with 7% uranyl acetate (Uranyl acetate; Polysciences, US) in methanol and pH 12 lead citrate (Lead citrate; Polysciences, US) [9,11,20] at room temperature for each 7 minutes. The samples were finally examined by TEM (JEM1010; JEOL, Japan).
Ten electron micrographs of the lower and middle stratum corneum from the five participants were taken at 30,000~250,000× magnification. The thickness (number of bilayers) and continuity of the lipid bilayer were graded for each photomicrograph using the following scale: 1, few or very patchy and interrupted lipid bilayers; 2, intermediate between 1 and 3; 3, moderate number of lipid bilayers or some interruption in the lipid bilayers; 4, intermediate between 3 and 5; 5, high number of lipid bilayers or continuous uninterrupted lipid bilayers. Mean scores for thickness and continuity in the ten photomicrographs were calculated, and the values of these two measurements were then added together. Corneocyte arrangement was assessed according to the following scale: 1, very disorganized arrangement and widened intercellular space; 2, intermediate between 1 and 3; 3, mild disorganization and widened intercellular space; 4, intermediate between 3 and 5; 5, organized arrangement and constant intercellular space.
Statistical analyses
Statistical analyses were conducted using PASW Statistics software, version 18.0 (SPSS, USA). Repeated measures ANOVA tests were used to compare changes in TEWL, skin hydration, PICAD scores, and CADESI values over time. A Wilcoxon singled-rank test was performed to compare changes in TEM scores. P values <0.05 were considered statistically significant.
Results
Lesion (CADESI) and pruritus scores
The modified CADESI and PICAD scores decreased significantly over time. The mean modified CADESI score for all participants decreased from 189.650 to 164.700 after 2 weeks, and was further reduced to 118.650 after 4 weeks (Fig. 1A). Similarly, the mean PICAD score for all participants decreased from 32.850 to 28.800 after 2 weeks, and fell to 21.000 after 4 weeks (Fig. 1B). Taken together, these data indicate that use of a moisturizer containing ceramide resulted in significant improvement of CAD symptoms.
TEWL and skin hydration
Functional measurements of CAD severity (i.e. TEWL and skin hydration) also improved significantly after application of the moisturizer. The mean TEWL score decreased from 32.535 to 28.555 after 2 weeks, and fell to 24.730 after 4 weeks (Fig. 1C). The mean skin hydration score increased rapidly from 2.626 to 12.810 after 2 weeks, and subsequently increased to 29.900 after 4 weeks (Fig. 1D).
TEM measurements
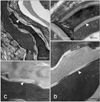
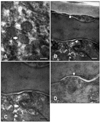
Lipid deposition in the stratum corneum and corneocyte integrity were increased in the five dogs evaluated by TEM (Fig. 1E). With untreated CAD, the corneocytes were arranged in a disorganized manner and were less compact with wider intercellular spaces (Fig. 2A). The lipid lamellae were also greatly disorganized and occupied the reduced intercorneocyte space (Figs. 3A and C). After treatment with the moisturizer, corneocyte organization became more regular and compact in the stratum corneum (Fig. 2B). Similarly, the lipid lamellae were more organized and more of the intercorneocyte space was occupied by the nearly normal lipid bilayer (Figs. 3B and D). At the junction between the stratum granulosum and stratum corneum, the lamellar body was composed of lipid lamellar disks (Fig. 4A). Their contents were actively extruded into the intercellular space and the disks were reorganized (Figs. 4B, C, and D).
Discussion
Recently, there has been increasing evidence demonstrating that the skin barrier function may play an important role in CAD pathogenesis [12,13,18]. Skin barrier function is dependent on the lower part of the stratum corneum and integrity of this structure. Stratum corneum integrity is maintained by keratinocytes and desmosomes along with intercellular ceramides, fatty acids, and cholesterol [5,6]. In previous electron microscopy studies, intercellular lipid deposition in the stratrum corneum of atopic canine skin was found to be markedly heterogeneous compared to that of normal canine skin [9]. Many intercellular spaces were devoid of lipid, and intercellular lipid lamellae (when present) usually exhibited an abnormal and incomplete structure. In a study of intercellular lipid composition using thin-layer chromatography, it was demonstrated that the levels of ceramide 1 and ceramide 9 were decreased in the non-lesional skin of atopic dogs compared to healthy controls [23]. A recent study also reported that non-lesional skin of atopic dogs had disorganized and reduced lamellar lipids [20]. Application of a topical skin lipid complex resulted in increased production of endogenous lipids, leading to improved skin barrier function.
The proper use of moisturizers is the most useful and important step for managing AD by reducing the risks of cracking and improving skin hydration. Moisturizers containing physiologic lipids can also improve the barrier function [25]. Due to ceramide deficiency in canine atopic skin, moisturizers with ceramide are expected to be more beneficial for treating CAD. However, it has been difficult to increase the skin concentration of ceramides due to the gelling phenomenon. To prevent the gelling phenomenon in complex formulations, a new system was designed. A physiologic lipid mixture containing ceramide, cholesterol, and fatty acids in an optimal ratio was formed into lipid granules using the fluid bed technique [7]. Many clinical studies evaluating the efficacy of the lipid complex for treating AD in humans have shown that the complex was beneficial alone or in combination with other agents [2,16,19]. On the other hand, there is lack of studies evaluating the effect of physiologic lipid complexes on CAD.
Our study showed that the moisturizer containing physiologic lipids can be helpful for managing CAD and promoting skin barrier repair. Our TEM results indicated that the disorganized and abnormal lipid lamellae in the stratum corneum were repaired after applying the ceramide-based moisturizer. Additionally, corneocyte arrangement improved although it was not completely restored. All parameters used to evaluate CAD severity, TEWL, skin hydration, and PICAD and CADESI scores were gradually improved. In particular, skin hydration and PICAD scores improved dramatically. Substantial PICAD improvement may be very significant for atopic patients due to the huge contribution of this factor to the quality of life. Although further evaluation is necessary, this study demonstrated that a moisturizer containing physiologic lipids is more effective for improving skin hydration than more conventional emollients. Therefore, the moisturizer we tested could be used as an adjunctive therapy for treating CAD. Moreover, none of the canine participants in the current study experienced adverse effects attributed to the moisturizer.
In addition to physiologic lipids, the moisturizer also contained panduratin that has both antimicrobial and anti-inflammatory effects. Atopic patients are vulnerable to bacterial skin infections, especially ones caused by Staphylococcus species. Therefore, it is hypothesized that panduratin may have helped improve atopic symptoms in this study.
The current investigation has some limitations. First, it was impossible to apply the moisturizer to the extremities and head due to its creamy texture. Therefore, we could not evaluate its efficacy in areas where many atopic patients have lesions. Second, comparisons were not made with other treatments that are known to be effective for managing CAD. Further studies comparing the effects of the moisturizer containing physiologic lipids to other treatments may offer additional information about the effects of physiologic lipids. If the moisturizer formulation were modified for used on haired dogs, the availability of this product would be further increased.
This study showed that application of ceramide-based moisturizer improved TEWL, PICAD, CADESI values and hydration states dramatically in dogs with atopic dermatitis. Electron micrographs also showed that corneocytes and lipid lamellae which usually disorganized in dogs with atopic dermatitis became regularly organized after application of the moisturizer. In conclusion, it is expected that the application of a moisturizer containing physiologic lipids can restore the lipid bilayer of the stratum corneum and this may ameliorate clinical symptoms associated with CAD.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download