Abstract
Recent studies have shown that mesenchymal stem cells (MSCs) are able to differentiate into multi-lineage cells such as adipocytes, chondroblasts, and osteoblasts. Amniotic membrane from whole placenta is a good source of stem cells in humans. This membrane can potentially be used for wound healing and corneal surface reconstruction. Moreover, it can be easily obtained after delivery and is usually discarded as classified waste. In the present study, we successfully isolated and characterized equine amniotic membrane-derived mesenchymal stem cells (eAM-MSCs) that were cultured and maintained in low glucose Dulbecco's modified Eagle's medium. The proliferation of eAM-MSCs was measured based on the cumulative population doubling level (CPDL). Immunophenotyping of eAM-MSCs by flow cytometry showed that the major population was of mesenchymal origin. To confirm differentiation potential, a multi-lineage differentiation assay was conducted. We found that under appropriate conditions, eAM-MSCs are capable of multi-lineage differentiation. Our results indicated that eAM-MSCs may be a good source of stem cells, making them potentially useful for veterinary regenerative medicine and cell-based therapy.
The amniotic membrane, or amnion, is a layer of fetal membrane and one of the three placental layers [22]. During pregnancy, the placenta supports fetal development by supplying nutrients and oxygen to the uterine environment. Some reports have shown that the amniotic membrane can be used for healing wounds and corneal damage [22]. However, this membrane is usually treated as medical waste and discarded.
Mesenchymal stem cells (MSCs), also known as multipotent stem cells or mesenchymal stromal cells, have unique characteristics such as a fibroblast-like morphology, expression of specific surface markers, and multipotent differentiation capacity [5,12]. Human MSCs can be isolated from various tissues including bone marrow, adipose tissue, umbilical cord blood, Wharton's jelly, amniotic fluid, and placenta [2,8,10,13,16,18]. Additionally, many groups have isolated and characterized MSCs recovered from human amniotic membranes [5,15,17].
In equines, several reports have demonstrated that isolation and characterization of stem cells could be possible using equine-specific tissues such as bone marrow, umbilical cord blood, and adipose tissue [1,3,11]. Some studies examining transplantation with equine stem cells for therapeutic purposes in various experiment models of articular defect, tendinitis, and osteoarthritis have also been conducted [11,19,24,27]. However, it is accompanied methodologically surgical and invasive procedure to harvest stem cell source from bone marrow and adipose tissue [1,3], which method could cause suffering from animals. Umbilical cord blood can serve as a source of stem cells without the need for painful harvesting methodologies. Nevertheless, studies of stem cells from umbilical cord blood are inadequate compared to ones evaluating stem cells from bone marrow and adipose tissue [4]. Similarly, amniotic membrane could also serve as a source of stem cells that are easily obtained on a large scale with minimal pain and suffering.
In the present study, we successfully isolated and characterized equine amniotic membrane-derived MSCs (eAM-MSCs), and showed that these cells are capable of self-renewal and multi-lineage differentiation. Additionally, the cells proliferated vigorously and displayed a similar morphology to that typical of human MSCs. Therefore, we suggest that eAM may be a useful source of stem cells for veterinary cell-based therapy and regenerative medicine.
All tissues were obtained via normal delivery without the use of any invasive methodologies. We used amniotic membranes that are normally discarded after delivery. The amnion samples were provided free of charge for the purposes of our study. The isolated membranes were only used for stem cell isolation and characterization. All safety compliances were strictly observed and the study adhered to the Policy and Regulation for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Seoul National University, Korea).
All placental samples (n = 4) were obtained after parturition from Thoroughbred mares at a private horse farm in Korea. To prevent contamination and damage to the tissues, all samples were collected immediately after parturition using sterilized surgical equipment. The collected placenta samples were stored at 4℃ and transported to the laboratory as quickly as possible. The amniotic membranes were mechanically peeled away from the allantois.
Cell isolation and culturing were performed as previously described with some modifications [5,21]. In brief, the collected amniotic membranes were washed with normal saline (0.9%) three~four times under sterile conditions to remove debris and blood. To detach and remove the epithelial layer from the amniotic membranes, the membranes were treated with 0.05% trypsin for 1 h at 37℃. After trypsinization, the membranes were washed with normal saline (0.9%) three~four times. After washing, the amniotic membrane was minced with a surgical blade and scissors. The minced tissue was digested at 37℃ for approximately 3~4 h with collagenase type I (2 mg/mL; Worthington Biochemical, USA). The digested samples were washed in phosphate-buffered saline (PBS; Corning, USA) and centrifuged at 350 × g for 5 min. The cell pellet was resuspended in basal culture medium composed of low glucose Dulbecco's modified Eagle's medium (LG-DMEM; Invitrogen, USA) containing 10% fetal bovine serum (FBS; Invitrogen, USA). The cells were cultured in a humidified atmosphere with 5% CO2. The basal culture medium was changed three times a week and passaged after reaching 80~90% confluency.
A cell proliferation assay was performed as previously described with some modifications [20]. Estimated growth efficiency and proliferation potential of the eAM-MSCs were determined based on the total CPDL using the formula CPDL = ln (Nf / Ni) ln2, where Ni is the initial number of cells seeded, Nf is the final number of harvested cells, and ln is the natural log. Cells (5 × 104) were plated in triplicate on a 6-well culture plate (Nalge Nunc International, USA) and subcultured for 5~7 days. The cells were then counted and 5 × 104 cells were re-plated. To determine the CPDL, the population doubling for each passage was calculated and then added to the population doubling levels of the previous passages.
To detect any chromosomal abnormalities in the eAM-MSCs, a karyotype analysis was conducted followed by a standard Q-band analysis at passage 5. Briefly, mitosis was inhibited in eAM-MSCs with 0.1 µg/mL colchicine (Sigma) for 20 min. The cells were then suspended in a hypotonic solution (0.075M KCl) and incubated for 20 min at 37℃. Next, the cells were pelleted at 1,000 rpm for 10 min and fixed by washing three times in methanol : glacial acetic acid (3 : 1). Chromosome spreads were obtained by pipetting the cell suspension onto clean glass slides and air-dried. Metaphases were captured with a CCD camera (Olympus, Japan), the chromosomes were counted, and banding patterns were analyzed.
Cells were stained for flow cytometry with specific antibodies following the supplier's protocol (BD Biosciences, USA). In brief, cultured eAM-MSCs were washed twice in PBS and harvested using 0.25% trypsin/EDTA. The cells were then washed with PBS and divided into groups for antibody staining. Each aliquot contained approximately 1 × 105 cells. The following antibodies were used to detect cell surface antigens: mouse anti-human CD19, mouse anti-human CD20, mouse anti-human CD28, mouse anti-human CD31, mouse anti-human CD34, mouse anti-human CD38, mouse anti-human CD41a, mouse anti-human CD44, mouse anti-human CD62L, mouse anti-human CD62P, mouse anti-human CD90, mouse anti-human CD200 (BD Biosciences, USA), and mouse anti-human CD105 (AbD Serotec, USA). All antibodies were conjugated with fluorescein isothiocyanate or phycoerythrin. The cells were stained for 30 min at 4℃. After incubation, the cells were washed with PBS and re-suspended in 500 µL of PBS. Analysis was performed with a FACS Calibur flow cytometer (BD Biosciences, USA) and Cell Quest Pro software (BD Biosciences, USA).
To test for osteogenic capacity, eAM-MSCs were treated with osteogenic differentiation medium containing ascorbic acid 2-phosphate (50 µM), dexamethasone (100 nM), β-glycerophosphate (10 mM; Sigma-Aldrich, USA), and 10% FBS in LG-DMEM. The cells (1 × 105) were plated in triplicate on 6-well plates. When the cells were 80~90% confluent, the culture medium was changed to the osteogenic differentiation medium and the cells were incubated for 3 weeks. The differentiation medium was changed twice weekly. After differentiation, the cells were stained with Alizarin Red S and von Kossa reagent to detect calcium deposition. For Alizarin Red S staining, the cells were washed with PBS and fixed with ice-cold 70% ethanol for 1 h at 4℃. Next, the cells were rinsed three to four times with distilled water and stained with Alizarin Red S (40 mM, pH 4.2; Sigma-Aldrich, USA) for 10 min at room temperature. To remove excess dye, the cells were rinsed with distilled water. Cells stained with Alizarin Red S were solubilized in cetylpyridinium chloride (100 mM; Sigma-Aldrich, USA) for 1 h. Absorbance of the solubilized cells was measured at 570 nm using a spectrophotometer [20]. For von Kossa staining, the cells were stained with 5% silver nitrate for 30 to 60 min while exposed to ultraviolet light, incubated with 5% sodium thiosulfate for 2 to 3 min, and then counterstained with Nuclear Red Stain for 5 min.
To test for adipogenic differentiation capacity, eAM-MSCs were treated with adipogenic differentiation medium composed of dexamethasone (1 µM), indomethacin (60 µM), 3-isobutyl-1-metylxanthine (500 µM), insulin (5 µg/mL; Sigma-Aldrich, USA), and 10% FBS in LG-DMEM for 3 weeks after reaching 80~90% confluency. The medium was replaced twice weekly. After differentiation, Oil Red O staining was conducted to confirm the formation of lipid inclusions. The cells were fixed in 10% formalin for at least 1 h and rinsed with 60% isopropanol prior to incubation in freshly diluted Oil Red O for 10 min. Stains were solubilized with 100% isopropanol, and the resulting absorbance was measured at 500 nm using a spectrophotometer [20].
To test for chondrogenic capacity, eAM-MSCs were treated with chondrogenic differentiation medium. The cells (5 × 105) were transferred to a 15-mL polypropylene tube and pelleted. The pellets were cultured in 1 mL of chondrogenic differentiation medium (Lonza, Switzerland) for 3 weeks. The medium was changed three times weekly. After differentiation, the pellets were embedded in paraffin and 3-µm sections were cut. To detect chondrogenesis, the sections were stained with toluidine blue and Alcian blue-PAS following standard protocols [20]. In brief, a 3-µm cell pellet section mounted on a slide was deparaffinized and hydrated with distilled water. For toluidine blue staining, the slide was immersed in a toluidine blue working solution for 1 min. Excess unbound stain was removed by several washes with distilled water. The slide was quickly dehydrated with sequential washes of 95% and absolute alcohol. For Alcian blue-PAS staining, the slide was stained with Alcian blue (pH 2.5), 0.5% periodic acid, and Schiff's reagent. The slide was cleared with xylene and covered with Canada balsam and a coverslip.
Placenta tissues were collected from mares after parturition. The collected tissues were quickly transported to the laboratory to prevent possible contamination from the dusty environment of the stall where the horses were housed. Under sterile condition, we peeled the amniotic membrane away from the placenta tissue using surgical instruments (Fig. 1A). We isolated and cultured eAM-MSCs with a fibroblast-like and spindle morphology typical of MSCs, and that adhered to the plastic culture dish surface (Figs. 1B and C). To measure the proliferation potential of the isolated eAM-MSCs, we calculated the CPDL. eAM-MSCs (5 × 104 cells/well) were seeded in a 6-well culture plate and subcultured 5~7 days later. The procedure was repeated until passage 14 to measuring the CPDL. The stable increasing graph of cell growth was observed (Fig. 1D). The cells were also confirmed to have a normal karyotype with 64 chromosomes (Fig. 1E).
We conducted flow cytometric analysis of the eAM-MSCs at passage 5 and monitored the expression of 13 CD markers (CD19, CD20, CD28, CD31, CD34, CD38, CD41a, CD44, CD62L, CD62P, CD90, CD105, and CD200) to determine whether the cells displayed an MSC phenotype (Fig. 2). The eAM-MSCs expressed CD44, CD90 and CD105. CD90, also known as Thy-1, is a marker for various types of stem cells including hepatic stem cells, keratinocyte stem cells, endometrial stem cells, and MSCs. CD105, which is also called SH2, is a well-known MSC marker. Other markers such as those expressed by immune cells (CD19, CD20, CD28, CD38, CD62L and CD200), endothelial cells (CD31 and CD62P), hematopoietic cells (CD34), and platelets (CD41a) were not found.
Calcium mineralization of eAM-MSCs treated with osteogenic induction medium was detected by Alizarin Red S or von Kossa staining, and indicated osteogenesis. Cells grown in basal culture medium alone were used as a negative control. After osteogenic differentiation, positive and strong Alizarin Red S and von Kossa staining was detected (Figs. 3C, D, G, and H). Under basal culture conditions, the cells were negative for Alizarin Red S and von Kossa staining (Figs. 3A, B, E, and F). For quantification, cells stained with Alizarin Red S were solubilized with 100 mM cetylpyridinium chloride and the absorbance was measured. Compared to the negative control, absorbance for the differentiated cells was approximately 15-fold greater (Fig. 3I).
To confirm the ability of eAM-MSCs to undergo adipogenesis, the cells were treated with adipogenic induction medium that was changed every 3 days for 3 weeks. After differentiation, the cells were stained with Oil Red O to detect the formation of lipid inclusions. Under differentiation conditions, lipid droplets stained with Oil Red O were observed (Figs. 4C and D). Black arrows in Fig. 4D indicate the stained lipid droplets. No Oil Red O stating was observed in cells grown under basal culture medium that were used as a negative control (Figs 4A and B). For quantification, the cells were solubilized with 100% isopropanol and the absorbance was measured. Absorbance for the differentiated cells was approximately five-times greater than that for the negative control (Fig. 4E).
To confirm the chondrogenesis potential of the eAM-MSCs, the cells were seeded into 15-mL polypropylene tubes and pelleted. The pellets were incubated at 37℃ in a 5% CO2 incubator with chondrogenic induction medium that was changed every 3 days for 3 weeks. After differentiation, we observed the formation of pellets in the bottom of the polypropylene tube. The pellets formed an ovoid and opaque structure (Figs. 5A and B). The black arrow indicates the pellet formation (Fig. 5A). Pellet formation was not observed for cells grown under control conditions. The control cells dispersed when the medium was changed, indicating that chondrogenesis had not occurred with the basal culture medium. The pellet was positive for staining with toluidine blue (Fig. 5C) and Alcian blue-PAS (Fig. 5D).
The placenta, which is of both fetal and maternal origin, is round and has numerous blood vessels, grows throughout pregnancy, and consist of three membrane layers. Functions of the placenta include transporting nutrients from the mother to the fetus, allowing waste elimination, and facilitating gas exchange. These functions are essential for fetal survival and development [28]. The amnion is the innermost of the two fetal membranes. It encloses the fetus, allowing it to move freely and providing protection from the external environment. The amniotic membrane has a thin, nonvascular structure with two layers: an epithelial monolayer and stromal layer [28].
After parturition, the placenta is classified as medical waste and usually discarded. There are, however, clinical studies showing that the amniotic membrane can potentially be used to promote wound healing and corneal reconstruction [9,25]. Additionally, isolation and characterization of human MSCs from the amniotic membrane and whole placenta have also been reported [22]. These stem cells have tri-lineage differentiation and self-renewal capabilities, which are characteristic MSC features. MSCs have many advantages for use in cell-based therapy including immune privilege, an absence of associated ethical issues, and no requirement of invasive procedures for harvesting the amnion. Recently, equine-derived stem cells were isolated and their potential use in cell-based therapy was examined [7,19,24,27]. However, these investigations have been confined to limited stem cell sources such as equine adipose tissue, umbilical cord blood, and bone marrow [1,3,11]. Therefore, a greater diversity of stem cell sources is required.
In the present study, we successfully isolated and characterized MSCs from eAM. The collected amniotic membrane was enzymatically digested to recover the cells. After digestion, the eAM-MSCs were seeded in basal culture medium until passage 14. Using this procedure, we obtained cells from four equine amniotic tissue samples. The rate of success was 100%, and all isolated cells (from all four samples) showed similar morphologies. The cells were subcultured on the surface of plastic culture dishes. A single cell line was randomly selected. All experiments including CDPL determination, flow cytometric analysis, and differentiation studies were conducted using only the selected cell line (in triplicate).
A consistent level of cell proliferation is a distinct characteristic of stem cells. These cells have a self-renewal ability related to continuous and steady proliferation. Our results demonstrated that the isolated eAM-MSCs are capable of self-renewal, a typical feature of mesenchymal cells. Furthermore, the cells were fibroblast-like, adhered to the plastic culture dish surface, and possessed a normal karyotype. Due to their robust proliferation, the eAM-MSC may be sufficient for in vivo applications.
Immunophenotype characterization indicated that the eAM-MSCs were positive for MSCs markers such as CD44 (98.98%), CD90 (100%), and CD105 (100%). CD44 is a cell-surface glycoprotein that plays a role in MSC migration [23]. MSCs display a distinctive pattern of cell surface antigens such as CD44, CD90, and CD105. Antigens that are not typically found on MSCs include CD11b, CD14, CD19, CD79a, CD34, CD45, and HLA-DR [6]. Our results demonstrated that eAM-MSCs have an immunophenotype similar to that of a typical MSC. Since there are no equine-specific antibodies appropriate for flow cytometry, we used human host antibodies that have cross-reactivity among different species [20]. Our previous study showed a similarity between Equus caballus and Homo sapiens CD markers by comparing amino acid sequences. All CD markers used for the present study have amino acid sequences suitable for cross-reactivity (56~84%). For MSC characterization using flow cytometry, it is essential to confirm the expression levels of CD45 and CD73. While, identity of CD45 and CD73 were not investigated since reference sequence of Equus caballus did not exist [20]. A previous study also examined equine surface antigen expression using anti-rat antibodies based on the same principle of interspecies cross-reactivity [3]. Although these data demonstrated the high degree of similarity between Equus caballus and Homo sapiens CD markers, more equine-specific antibodies for flow cytometry should be identified to confirm the identity of equine-derived stem cells.
In addition, we demonstrated the multipotent differentiation capability of eAM-MSCs. We found that eAM-MSCs differentiated into osteocytes, adipocytes, and chondrocytes using specific culture media. eAM-MSCs grown under osteogenic differentiation conditions acquired calcium deposits identified with Alizarin Red S and von Kossa staining. Calcium accumulation was not observed in cells cultured under control conditions. In the adipogenic differentiation study, lipid droplets were observed in the cells after 3 weeks of differentiation. To confirm adipogenic differentiation, we performed Oil Red O staining. Differentiated cells were positive for Oil Red O staining although only a few cells were stained and contained fat droplets. We tried to increase the adipogenic efficiency with various methods but could not observe any difference (data not shown). It might be that eAM-MSCS differentiation favored the osteogenic axis rather than the adipogenic axis. Findings similar to the ones from our study were reported in animal tissue-derived stem cells [20,26], and it could be that osteogenesis and adipogenesis are achieved through competing pathways [14]. In the chondrogenic differentiation study, cells that were cultured in chondrogenic differentiation medium aggregated and formed pellets unlike cells that were grown in the control medium. The differentiated cells were also positive for toluidine blue and Alcian blue-PAS staining.
For clinical cell-based therapies, large numbers of stem cells from diverse sources are required. In large animals such as horses, two major barriers for clinical cell therapy are the limited number of stem cell sources and difficulties in identifying stem cells. In the current study, we isolated stem cells from eAM. These cells were found to have an immunophenotype characteristic of MSCs and are capable of self-renewal. Moreover, the rate of eAM-MSC proliferation was stable until passage 14. Taken together, these findings suggest that eAM-MSCs are a potential source of stem cells and could be useful for regenerative medicine in horses.
Figures and Tables
 | Fig. 1Primary culturing of equine amniotic membrane-derived mesenchymal stem cells (eAM-MSCs) and determination of the cumulative population doubling level (CPDL). (A) Harvesting of eAM tissue. (B and C) Phase contrast images of eAM-MSCs. The cells were cultured in low glucose Dulbecco's modified Eagle's medium (LG-DMEM) with 10% FBS. The cells had a spindle morphology with a fibroblast-like structure similar to that of human MSCs. Scale bars = 50 µm. (D) Cell growth curve of the eAM-MSCs. The CPDL was measured from passage 3 to passage 14, and evaluated as described in the Materials and Methods section. Cells grew consistently until passage 14. (E) Karyotype of eAM-MSCs at passage5 showing a euploid number of chromosomes. |
 | Fig. 2Flow cytometry analysis of eAM-MSCs. The analysis was performed at passage 5. Values show the signal intensity of the indicated antigen. |
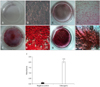 | Fig. 3Osteogenic differentiation of the eAM-MSCs. Negative control cells (A, B, E, and F) were grown in LG-DMEM with 10% FBS. No Alizarin Red S or von Kossa staining was observed. The cells (C, D, G and H) were also grown in osteogenic induction medium. The differentiated cells showed strong Alizarin Red S (C and D) and von Kossa (G and H) staining. Scale bars = 50 µm. For quantification, Alizarin Red S-stained cells were solubilized with 100 mM cetylpyridinium chloride and the absorbance was measured spectrophotometrically at 570 nm for 0.5 seconds (I). Compared to the negative control, absorbance for the differentiated cells was approximately 15-fold greater. (A~H) Alizarin Red S and von Kossa staining after 3 weeks of osteogenic induction or culturing under control conditions. All analyses were performed in triplicate and the mean ± standard deviation (SD) was plotted (***p < 0.001). |
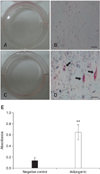 | Fig. 4Adipogenic differentiation of the eAM-MSCs. Negative control cells (A and B) were grown in LG-DMEM with 10% FBS. No Oil Red O staining was observed in the control cells. (C and D) Cells were also grown in adipogenic induction medium. Lipid droplets within the differentiated cells were strongly stained with Oil Red O. Black arrows indicate the fat droplets stained red. Scale bars = 50 µm. For quantification, stained cells were solubilized with 100% isopropanol, and absorbance was measured spectrophotometrically at 500 nm for 0.5 seconds (E). Compared to the negative control, absorbance of the differentiated cells was approximately 5-fold greater. (A~D) Oil Red O staining after 3 weeks of adipogenic induction. All analyses were performed in triplicate and the mean ± SD was plotted (**p < 0.01). |
 | Fig. 5Chondrogenic differentiation of the eAM-MSCs. After 3 weeks of chondrogenic induction, pellet formation was observed. (A) Formation of chondrogenic pellets occurred at the bottom of a 15-mL polypropylene tube. The black arrow indicates a pellet. (B) Image of an oval-shaped chondrogenic pellet. (C) Toluidine blue and (D) Alcian blue-PAS staining of the chondrogenic pellets. The pellets were embedded in paraffin and cut into 3-µm sections that were mounted on slides. The sections were stained with toluidine blue and Alcian blue-PAS. A typical cartilaginous tissue phenotype was observed. Scale bars = 100 µm. |
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST, 2010-0020265).
References
1. Arnhold SJ, Goletz I, Klein H, Stumpf G, Beluche LA, Rohde C, Addicks K, Litzke LF. Isolation and characterization of bone marrow-derived equine mesenchymal stem cells. Am J Vet Res. 2007; 68:1095–1105.

2. De Bruyn C, Najar M, Raicevic G, Meuleman N, Pieters K, Stamatopoulos B, Delforge A, Bron D, Lagneaux L. A rapid, simple, and reproducible method for the isolation of mesenchymal stromal cells from Wharton's jelly without enzymatic treatment. Stem Cells Dev. 2011; 20:547–557.

3. de Mattos Carvalho A, Alves ALG, Golim MA, Moroz A, Hussni CA, de Oliveira PGG, Deffune E. Isolation and immunophenotypic characterization of mesenchymal stem cells derived from equine species adipose tissue. Vet Immunol Immunopathol. 2009; 132:303–306.

4. De Schauwer C, Meyer E, Van de Walle GR, Van Soom A. Markers of stemness in equine mesenchymal stem cells: a plea for uniformity. Theriogenology. 2011; 75:1431–1443.

5. Díaz-Prado S, Muiños-López E, Hermida-Gómez T, Rendal-Vázquez ME, Fuentes-Boquete I, de Toro FJ, Blanco FJ. Isolation and characterization of mesenchymal stem cells from human amniotic membrane. Tissue Eng Part C Methods. 2011; 17:49–59.

6. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, Deans RJ, Keating A, Prockop DJ, Horwitz EM. The International Society for Cellular Therapy position statement. Minimal criteria for defining multipotent mesenchymal stromal cells. Cytotherapy. 2006; 8:315–317.

7. Frisbie DD, Kisiday JD, Kawcak CE, Werpy NM, McIlwraith CW. Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis. J Orthop Res. 2009; 27:1675–1680.

8. Kamishina H, Deng J, Oji T, Cheeseman JA, Clemmons RM. Expression of neural markers on bone marrow-derived canine mesenchymal stem cells. Am J Vet Res. 2006; 67:1921–1928.

9. Kesting MR, Wolff KD, Hohlweg-Majert B, Steinstraesser L. The role of allogenic amniotic membrane in burn treatment. J Burn Care Res. 2008; 29:907–916.

10. Klein JD, Fauza DO. Amniotic and placental mesenchymal stem cell isolation and culture. Methods Mol Biol. 2011; 698:75–88.

11. Koch TG, Heerkens T, Thomsen PD, Betts DH. Isolation of mesenchymal stem cells from equine umbilical cord blood. BMC Biotechnol. 2007; 7:26.

12. La Rocca G, Anzalone R, Corrao S, Magno F, Loria T, Lo Iacono M, Di Stefano A, Giannuzzi P, Marasa L, Cappello F, Zummo G, Farina F. Isolation and characterization of Oct-4+/HLA-G+ mesenchymal stem cells from human umbilical cord matrix: differentiation potential and detection of new markers. Histochem Cell Biol. 2009; 131:267–282.

13. Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004; 103:1669–1675.

14. Ling L, Nurcombe V, Cool SM. Wnt signaling controls the fate of mesenchymal stem cells. Gene. 2009; 433:1–7.

15. Marongiu F, Gramignoli R, Sun Q, Tahan V, Miki T, Dorko K, Ellis E, Strom SC. Isolation of amniotic mesenchymal stem cells. Curr Protoc Stem Cell Biol. 2010; 12:Suppl. 1E 5.1–1E 5.11.

16. Miao Z, Jin J, Chen L, Zhu J, Huang W, Zhao J, Qian H, Zhang X. Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biol Int. 2006; 30:681–687.

17. Mihu CM, Rus Ciucă D, Soritău O, Suşman S, Mihu D. Isolation and characterization of mesenchymal stem cells from the amniotic membrane. Rom J Morphol Embryol. 2009; 50:73–77.
18. Mosna F, Sensebé L, Krampera M. Human bone marrow and adipose tissue mesenchymal stem cells: a user's guide. Stem Cells Dev. 2010; 19:1449–1470.

19. Nixon AJ, Dahlgren LA, Haupt JL, Yeager AE, Ward DL. Effect of adipose-derived nucleated cell fractions on tendon repair in horses with collagenase-induced tendinitis. Am J Vet Res. 2008; 69:928–937.

20. Park SB, Seo MS, Kang JG, Chae JS, Kang KS. Isolation and characterization of equine amniotic fluid-derived multipotent stem cells. Cytotherapy. 2011; 13:341–349.

21. Park SB, Seo MS, Kim HS, Kang KS. Isolation and characterization of canine amniotic membrane-derived multipotent stem cells. PLoS One. 2012; 7:e44693.

22. Parolini O, Alviano F, Bagnara GP, Bilic G, Bühring HJ, Evangelista M, Hennerbichler S, Liu B, Magatti M, Mao N, Miki T, Marongiu F, Nakajima H, Nikaido T, Portmann-Lanz CB, Sankar V, Soncini M, Stadler G, Surbek D, Takahashi TA, Redl H, Sakuragawa N, Wolbank S, Zeisberger S, Zisch A, Strom SC. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells. 2008; 26:300–311.

23. Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CP, Wohlgemuth R. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008; 14:181–187.

24. Schnabel LV, Lynch ME, van der Meulen MCH, Yeager AE, Kornatowski MA, Nixon AJ. Mesenchymal stem cells and insulin-like growth factor-I gene-enhanced mesenchymal stem cells improve structural aspects of healing in equine flexor digitorum superficialis tendons. J Orthop Res. 2009; 27:1392–1398.

25. Takaoka M, Nakamura T, Sugai H, Bentley AJ, Nakajima N, Fullwood NJ, Yokoi N, Hyon SH, Kinoshita S. Sutureless amniotic membrane transplantation for ocular surface reconstruction with a chemically defined bioadhesive. Biomaterials. 2008; 29:2923–2931.

26. Vieira NM, Brandalise V, Zucconi E, Secco M, Strauss BE, Zatz M. Isolation, characterization, and differentiation potential of canine adipose-derived stem cells. Cell Transplant. 2010; 19:279–289.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download