Introduction
Meloxicam (MEL) is an anti-inflammatory drug commonly used to treat cattle [6]. This drug belongs to a general class of compounds known as non-steroidal anti-inflammatory drugs (NSAIDs). NSAIDs inhibit the activity of cyclooxygenase (COX), an enzyme that initiates prostanoid formation [25]. Analysis of the available literature has shown that current knowledge about the effect of MEL on bovine CD4+ lymphocytes is based on two publications. A single dose of the drug given to calves was not found to have any significant effect on the percentage of CD4+ cells in peripheral blood in our earlier study [18] nor in the one conducted by Bednarek et al. [2]. Likewise, we did not find MEL to exert any significant effect on the percentage of cells that were in an early stage of apoptosis. This is essentially the information that makes up the entire body of knowledge about the effects of MEL on bovine CD4+ lymphocytes.
Considering the number of reports showing that COX-2 helps regulate the production of interferon-γ (IFN-γ) [23], interleukin-10 (IL-10) [7], and transforming growth factor-β (TGF-β) [4,12], it would be extremely interesting and useful to understand the consequence of COX-2 inhibition on the production of these cytokines which are very important for regulating the immune response. The only available data showing the possible effect of MEL on cytokine production in cattle were published by Maeda et al. [14]. This study found that the drug did not significantly change the mRNA expression of IFN-γ, IL-2, or IL-4 in bovine peripheral blood mononuclear cells (PBMCs). There have been a few publications demonstrating the effects of MEL on the production of IFN-γ [20,21] and IL-10 [19,20]; however, these investigations were conducted with animals other than ruminants. Our preliminary research (unpublished data) showed that MEL does not equally affect the production of cytokines by different subpopulations of T cells. Therefore, we initiated comprehensive studies of this issue in CD4+ lymphocytes and have presented our findings in this paper.
It was also of interest to assess the effects of MEL on CD25+CD4+ cells and Foxp3 expression in these cells. In mice, T cells with a CD25+CD4+ phenotype are regulatory cells (Tregs) that play an important role in maintaining immunologic balance in the host by suppressing a wide variety of immune responses [24]. However, CD25 (the IL-2 receptor α chain) is not a specific Tregs marker because this factor is also expressed by activated T cells. Currently, the forkhead family transcription factor Foxp3 is the most specific marker used to distinguish regulatory cells (Foxp3+CD25+CD4+) from activated effector cells (Foxp3-CD25+CD4+) within CD25+CD4+ cell subpopulations [26]. However, it seems that the regulatory properties of bovine CD25+CD4+ cells are not equivalent to those of mouse lymphocytes with the same phenotype. Hoek et al. [8] were unable to demonstrate that bovine CD25highCD4+ and CD25lowCD4+ lymphocytes have suppressive properties using a functional assay, and concluded that these subpopulations in cattle are not composed of regulatory cells. Findings from our earlier research [17] are consistent with this conclusion since only a small percentage of bovine CD25highCD4+ and CD25lowCD4+ cells were found to express Foxp3, a factor which helps determine the regulatory properties of lymphocyte subpopulations.
There have been many recently published papers [3,22,27] on generating Foxp3+CD25+CD4+ regulatory cells using steroidal anti-inflammatory drugs (SAIDs). There have also been reports [1,9] indicating a possibility of inducing such cells with NSAIDs. We assumed that an absence of regulatory properties of bovine CD25highCD4+ and CD25lowCD4+ lymphocytes is a result of insufficient Foxp3 expression. Based on reports indicating the possibility of increasing Foxp3 expression in human or mouse CD25+CD4+ lymphocytes with SAIDs or NSAIDs, research was conducted to determine the effect of dexamethasone (a SAID) and MEL on the percentage of Foxp3+ cells among bovine CD25highCD4+ and CD25lowCD4+ lymphocytes, and on the number of cells in these subpopulations. We considered the possibility that inducing Foxp3 expression in CD25highCD4+ and CD25lowCD4+ cells may be one of the mechanisms underlying SAID and NSAID action that is responsible for some pharmacological effects of these drugs in cattle. We have shown [17] that dexamethasone increases the number of bovine CD25highCD4+ and CD25lowCD4+ lymphocytes but unlike in mice and humans, this drug reduces Foxp3 expression in these cells. This current study produced results demonstrating the effect of MEL on the numbers of bovine CD25highCD4+ and CD25lowCD4+ cells, and on the expression of Foxp3 in these cells.
Considering the potential effect that MEL may have on CD4+ cells and scarce knowledge on this issue with respect to cattle, the present study was conducted to determine whether MEL could affect the number of these cells. The effects of MEL on apoptosis, production of selected cytokines, and Foxp3 expression were also evaluated. Three subpopulations of CD4+ lymphocytes (CD25highCD4+, CD25lowCD4+, and CD25- CD4+) were used for our investigation.
Materials and Methods
Animals
This study was carried out on 36 clinically healthy heifers (Polish black and white) 12 months old. The animals were kept indoors and originated from a dairy farm located in Bałdy (Poland). The heifers were housed and treated in accordance with the rules approved by the Local Ethics Commission of the University of Warmia and Mazury in Olsztyn (Ethic Commission Opinion No. 82/2010).
Isolation of PBMCs and cell culture conditions
Blood was aseptically drawn by venipuncture from the jugular vein into sterile heparinized vacutainer tubes (BD Biosciences, USA). PBMCs were isolated with a Histopaque 1.077 (Sigma-Aldrich, USA) density gradient by centrifugation at 400 × g for 30 min at room temperature (RT). PBMCs were recovered from the interface, washed (300 × g for 10 min at 4℃; these parameters were used for all cell washing procedures) three times with complete medium [CM; RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 10 mM HEPES buffer, 10 mM nonessential amino acids, 10 mM sodium pyruvate, and 10 U/mL penicillin/streptomycin (all reagents were purchased from Sigma-Aldrich, USA)], and resuspended in CM.
For all experiments, the cells were treated with MEL (Sigma-Aldrich, USA) at a concentration reflecting plasma levels achieved in vivo with therapeutic doses of the drug (MEL 5 × 10-6 M) and a concentration 10 times lower (MEL 5 × 10-7 M). For phenotypic analysis and detection of apoptosis, PBMCs were diluted to a final concentration of 106 cells/mL in CM, seeded in 12-well plates (BD Falcon, USA) in 2-mL aliquots, and incubated for 12, 24, 48, and 168 h in the absence (control) or presence of MEL. Apoptosis was monitored after 12 and 24 h of incubation. To analyze the intracellular expression of IL-10, IFN-γ, and TGF-β, the PBMCs were diluted to 5 × 106 cells/mL in CM and seeded in 24-well plates (BD Falcon, USA) in 1-mL aliquots. The cells were pre-incubated for 1 h without (control) or with the two different concentrations MEL followed by stimulation for 6 h (to evaluate IL-10 and IFN-γ production) or 12 h (to evaluate TGF-β production) with 5 µg/mL concanavalin A (Con A; Sigma-Aldrich, USA) in the presence of 10 µg/mL brefeldin A (Sigma-Aldrich, USA) during the last 5 h. The plates were incubated at 37℃ in an atmosphere containing 5% CO2 and 95% air.
Flow cytometry
Extracellular staining
The cells were removed from the wells by pipetting and rinsing with FACS buffer [FB, 1 × Dulbecco's phosphate-buffered saline (DPBS) devoid of Ca2+ and Mg2+ with 2% (v/v) heat-inactivated FBS; both reagents were purchased from Sigma-Aldrich, USA], transferred into individual tubes (12 × 75 mm, BD Falcon, USA), and centrifuged. After washing in 2 mL FB, the cells were resuspended in 200 µL of FB and stained with FITC (fluorescein isothiocyanate) -conjugated mouse anti-bovine CD4 (1 : 20, CC8, IgG2a; AbD Serotec, UK) and PE(phycoerythrin)-conjugated mouse anti-bovine CD25 (1 : 200, IL-A111, IgG1; AbD Serotec, UK). After 45 min incubation on ice and in the dark, the cells were washed in 2 mL FB.
Intracellular staining for Foxp3
Cells stained for surface markers (as described above) were fixed by adding 100 µL leucoperm reagent A (AbD Serotec, UK) to each tube and incubating for 15 min at RT in the dark. Next, the cells were washed with 3 mL FB, permeabilized by adding 100 µL of leucoperm reagent B (AbD Serotec, UK), and subsequently stained with AF (alexa fluor) 647-conjugated human anti-bovine Foxp3 mAb (1 : 20, 7627, HuCAL Fab bivalent; AbD Serotec, UK) for 60 min at RT in the dark. The cells were then washed twice with 2 mL FB and analyzed by flow cytometry. An isotype control was made using the protocol described above except that the AF647-conjugated anti-Foxp3 mAb was replaced with hucal fab-dhlx-mh isotype control-AF647 (AbD Serotec, UK).
Staining for intracellular IL-10 and IFN-γ
Cells stained for surface markers (as described above) were fixed with 200 µL 2% paraformaldehyde in DPBS for 15 min on ice. After this, the samples were washed with 2 mL FB and then permeabilized with 2 mL 0.2% (w/v) saponin (Sigma-Aldrich, USA) in FB. The cells were subsequently stained with biotinylated mouse anti-bovine IL-10 mAb (1 : 1000, CC320, IgG1; AbD Serotec) for 45 min on ice in the dark and were then washed with 2 mL 0.2% saponin in FB. Next, the cells were stained with PerCP (peridinin-chlorophyll-protein complex) -conjugated streptavidin (1 : 400; BD Biosciences, USA) and AF647-conjugated mouse anti-bovine IFN-γ mAb (1 : 200, CC302, IgG1; AbD Serotec, UK) for 45 min on ice in the dark. Finally, the cells were washed twice with 2 mL FB and analyzed by flow cytometry. Isotype controls were made as described above except that the biotinylated mouse anti-bovine IL-10 and AF647-conjugated anti-IFN-γ mAb were replaced with biotinylated mouse IgG1 isotype control (AbD Serotec, UK) and AF647-conjugated mouse IgG1 isotype control (AbD Serotec, UK), respectively.
Staining for intracellular TGF-β
After extracellular staining (as described above) and fixation (200 µL 2% paraformaldehyde in Dulbecco's PBS per sample for 15 min on ice; both reagents were purchased from Sigma-Aldrich, USA), the cells were permeabilized with 2 mL SAP buffer [0.1% (w/v) saponin and 0.05% (w/v) NaN3 in Hanks' balanced salt solution (HBSS; all from Sigma-Aldrich, USA)] and stained with APC (allophycocyanin) -conjugated mouse anti-TGF-β mAb (1 : 20, 1D11, IgG1, this antibody reacts with human, mouse, and bovine TGF-β1 and TGF-β2; R&D Systems, USA) for 45 min at RT in the dark. The cells were then washed twice with 2 mL SAP buffer and analyzed by flow cytometry. An isotype control was made as described above except that the APC-conjugated anti-TGF-β mAb was replaced with APC-conjugated mouse IgG1 isotype control (R&D Systems, USA).
Apoptosis analysis
Cells stained for surface markers were washed once in 1 mL of 1 × annexin V binding buffer (BD Biosciences, USA). The supernatant was removed by centrifugation, and the cells were suspended in 100 µL of 1 × annexin V binding buffer. Next, 5 µL of APC-conjugated annexin V (BD Biosciences, USA) and 5 µL of 7-AAD (7-aminoactinomycin D; BD Biosciences, USA) were added to the cells. The cells were gently mixed, incubated for 15 min at RT in the dark, diluted with 400 µL of 1 × annexin V binding buffer, and analyzed by flow cytometry within 1 h.
FACS data acquisition and analysis
Flow cytometry was performed using a FACSCanto II cytometer (BD Biosciences, USA). Data were acquired with FACSDiva version 6.1.3 software (BD Biosciences, USA) and analyzed by FlowJo software (Tree Star, USA). Cytometry setup and tracking beads (CST; BD Biosciences, USA) were used to initialize the photomultiplier tube (PMT) settings. Unstained control cells as well as a single stain control for every fluorochrome were prepared and used to establish flow cytometric compensation. The absolute count represented the number of collected cells of each subset per sample taking into account that a fixed volume of sample (100 µL) was always analyzed. Values obtained in this manner would reflect changes in the absolute numbers of lymphocytes because (a) the same number of cells was seeded in each well, (b) total contents of each well were transferred to individual tubes after culturing, (c) the same volume of each sample (100 µL) was always analyzed by flow cytometry, and (d) all cytometry assays was performed using the same forward scatter (FSC) threshold. FlowJo software (Tree Star, USA) directly converted the percentage count of a cell subpopulation into an absolute count.
Statistical analysis
All data are presented as the mean ± standard error (SE). Student's unpaired t-test was used to compare groups. A p-value less than 0.05 was considered to be statistically significant. All data were analyzed with GraphPad Prism3 (GraphPad Software, USA) and graphed with SigmaPlot software (ver. 12; Systat Software, USA).
Results
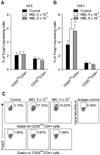
The effect of MEL on the percentage and absolute number of CD25highCD4+, CD25lowCD4+, and CD25CD4+ lymphocytes was evaluated after 12, 24, 48, and 168 h of exposure to the drug. It was found that MEL significantly [12 h: p < 0.01 (MEL 5 × 10-6), p < 0.05 (MEL 5 × 10-7); 24 h: p < 0.01] reduced the percentage of CD25highCD4+ cells (Fig. 1A and E) at 12 and 24 h; however, the absolute number of these cells was not significantly different from the control values. Exposure to MEL 5 × 10-6 for 48 and 168 h led to a considerable (p < 0.05) increase in the absolute number of CD25highCD4+ cells (Fig. 1B). However, a significant (p < 0.05) increase in the relative cell number was recorded only at 48 h after the treatment (Figs. 1A and E). MEL did not affect the absolute number of CD25-CD4+ lymphocytes (data not shown).
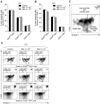
The influence of MEL on Foxp3 expression in CD25highCD4+, CD25lowCD4+, and CD4+CD25- cells was evaluated after 48 and 168 h of culturing in the presence or absence of the drug. Exposure to the drug for 48 h was not found to significantly affect the expression of Foxp3+ in the cell subpopulations (Fig. 2A). In contrast, prolonged (168 h) exposure of the PBMCs to MEL significantly (p < 0.05) increased the percentage of Foxp3+ cells among the CD25highCD4+ lymphocytes (Figs. 2B and C). No Foxp3+ cells were present among the CD4+CD25- cells in either the control or MEL-treated cultures (data not shown). The effect of MEL on apoptosis of CD25highCD4+, CD25lowCD4+, and CD25-CD4+ cells was evaluated after 12 and 24 h of incubation in the presence or absence of MEL. The drug was not found to have any significant effect on the percentage of cells undergoing early apoptosis among the CD4+ cell population (Fig. 3).
The effect of MEL on IL-10, TGF-β, and IFN-γ production was evaluated in PBMCs incubated with or without the drug and concurrent Con A stimulation in the presence of brefeldin A. MEL was not found to affect the percentage of IL-10+ or TGF-β+ cells in any of the lymphocyte subpopulations (Figs. 4A and B). MEL administered at the higher dose was shown to significantly increase (p < 0.05) the percentage of IFN-γ+ cells among CD4+CD25- lymphocytes. On the other hand, the production of this cytokine by CD25highCD4+ or CD25lowCD4+ cells was not significantly affected (Figs. 4C and D).
Discussion
Results of the present study showed that MEL did not deplete CD4+ lymphocyte populations, and could even increase the number of these cells relative to CD25highCD4+ cell populations. Despite temporary fluctuations in the relative number of CD25highCD4+ cells, MEL was not found to significantly affect the percentage of CD4+ lymphocytes (data not shown). This is consistent with our earlier findings [18] and those of other researchers [2] who observed that MEL administered at a single dose does not impact the percentage of CD4+ lymphocytes in calves. The absence of any negative effects of MEL on the absolute number of CD4+ lymphocytes concurs with the results of our evaluation showing the impact of MEL on apoptosis. We did not find MEL to have a significant effect on the percentage of cells in the early apoptotic stage in any of the subpopulations we assessed. Our previous investigation conducted on calves [18] also demonstrated that MEL does not alter the percentage of early apoptotic cells among CD4+ lymphocytes. An increase in the percentage of late apoptotic/necrotic cells among the CD4+ lymphocytes was observed after 24 h of exposure to the drug, but the increase was relatively small (the average difference between the drug-treated animals and the control group was about 1.5%) and short-term. It was thus doubtful that this effect was clinically relevant. Apart from our research, there has only been one report on the effect of MEL on lymphocyte mortality although the study focused on B cells. In this investigation, Kobayashi et al. [11] did not observe a decrease in the viability of normal B lymphocytes treated with etodolac or MEL.
After 12 and 24 h of incubation with MEL, a relatively small but statistically significant reduction of CD25highCD4+ lymphocyte percentage was found. However, this was not reflected by the absolute numbers. This disturbance was temporary and quickly resolved in that the percentage of CD25highCD4+ after 48 h of drug exposure was the same as the control values (MEL 5 × 10-7) or even higher (MEL 5 × 10-6). Moreover, cultures treated with MEL 5 × 10-6 for 48 and 168 h were observed to contain more CD25highCD4+ lymphocytes in terms of absolute numbers. These findings indicate that this phenomenon was not due to the pro- or anti-apoptotic effect of MEL on CD25highCD4+, CD25lowCD4+, or CD25-CD4+ cells. Moreover, our latest study [16] provides evidence suggesting that MEL does not affect the proliferation of cells belonging to these subsets. Thus, alterations in the percentage and absolute number of CD25highCD4+ cells observed in the present study could be caused by MEL-induced changes in the expression of CD25 molecules. It could be also speculated that MEL initially down-regulates and subsequently up-regulates CD25 expression on bovine CD4+ lymphocytes. Moreover, results obtained in the present study suggest that this later effect is dose-dependent and requires a MEL concentration achieved in vivo after therapeutic doses of the drug (0.5 mg/kg, MEL 5 × 10-6 M) are administered. This hypothesis requires further study.
Next, the nature of MEL-induced CD25highCD4+ lymphocytes was addressed. Our previous research [17] demonstrated that dexamethasone increases the absolute number of CD25highCD4+ cells although this increase was several times higher than that induced by MEL. This has been interpreted as an increase in the number of activated rather than regulatory lymphocytes due to the fact that dexamethasone significantly reduces the percentage of Foxp3+ cells in the CD25highCD4+ lymphocyte subpopulation. The situation was different with MEL. One cannot rule out the possibility that CD25highCD4+ lymphocytes generated in response to MEL 5 × 10-6 have regulatory properties since our current research revealed that prolonged exposure of PBMCs to MEL increased the percentage of Foxp3+ cells in the CD25highCD4+ subpopulation. Considering the fact that treatment with MEL 5 × 10-6 increased the absolute number of CD25highCD4+ lymphocytes, it should be assumed that the drug also increased the absolute number of Foxp3+CD25highCD4+ cells. Theoretically, one could conclude that MEL can promote the generation of inducible Tregs (iTregs) in cattle, but it seems that such a conclusion would be premature and far-fetched. It must be pointed out that the number of Foxp3+CD25+CD4+ cells in cattle is relatively small and increases induced by MEL were rather modest despite being statistically significant. Therefore, it is not certain whether such an effect is clinically relevant.
Considering the data presented above, it is obvious that caution should be exercised when interpreting the results indicating the effect of MEL on Foxp3+CD25highCD4+ cells. It should be emphasised that the current literature is not helpful for interpreting results from the present study because the effects of MEL on CD25+CD4+ cells or Foxp3 expression remain unclear. These issues have been examined with regard to other NSAIDs [1,9,13,28] or other substances acting as COX-2 inhibitors [29], but many of the results are contradictory. In line with our findings, Atarashi et al. [1] reported that ketoprofen completely inhibits prostaglandin E2 (PGE2) production and simultaneously induces Foxp3 expression in lymph node cells, indicating an inverse correlation between PGE2 production and Foxp3 expression. Other investigators [9] have demonstrated that acetyl-salicylic acid (ASA), another NSAID, significantly increases the relative levels of CD4+CD25+Foxp3+ Tregs cells among CD4+ cells in the periphery and thymus of mice. However, these studies did not prove that the drug-induced cells with a Treg phenotype have, in fact, a regulatory effect. Nevertheless, it should be emphasized that there are reasons to believe that the cells generated via NSAID action may have regulatory potential. It has been demonstrated [1] in mice that skin application of ketoprofen systemically suppresses contact hypersensitivity to picryl chloride by increasing CD25+CD4+ regulatory cell numbers. Moreover, Yokoyama et al. [29] found that the COX-2 inhibitor NS-398 promotes the generation of CD4+ regulatory cells and has an immunomodulatory effect that induces the indefinite survival of cardiac allografts. Completely opposite results and conclusions were reported by other groups [13,15,28]. Lönnroth et al. [13] demonstrated a decrease in the levels of molecules associated with immunosuppressive Treg cells, such as Foxp3 and IL-10, in tumor tissue from indomethacin-treated patients. Another study by Mahic et al. [15] found that the mechanism of CD25+CD4+ iTregs cell production depends on COX-2 expression and PGE2 secretion. This group concluded that PGE2 acts in a paracrine manner to inhibit effector T cell responses, and exerts autocrine effects on iTregs cells that lead to increased levels of intracellular cAMP and up-regulated Foxp3 expression. Moreover, other results of this team [28] suggest that iTregs cells contribute to the formation of an immunosuppressive microenvironment in colorectal cancer (CRC) and inhibit effector T cells through a COX-2/PGE2-dependent mechanism, thereby facilitating tumor growth. It was shown in this previous study that the COX inhibitor indomethacin significantly improves anti-tumor immune activity. Thus, it was concluded that therapeutic strategies targeting Treg cells and the PGE2-cAMP pathway may be useful for enhancing anti-tumor immune activity in CRC patients. However, there are contradictory data on the consequences of blocking COX-2 activity.
In the present study, the duration of exposure to MEL was highly correlated to in vivo conditions. The half-life of MEL in cattle is extremely long as previously shown by Coetzee et al. [5]. Researchers have found that the average half-life of MEL after a single oral administration is 27.54 h with a range (for different animals) from 19.97 to 44.29 h. The drug concentration decreases 10 times relative to the maximum concentration after about 100 h following drug administration. One should also take into account the fact that in cattle the drug is administered a second time several days after the first dose if needed.
The current study showed that MEL used at a higher dose increased IFN-γ production by CD25-CD4+ lymphocytes while the drug did not affect the percentage of IFN-γ+ cells within the CD25highCD4+ and CD25lowCD4+ cell subpopulations. Thus, MEL-induced increases of IFN-γ production in bovine CD4+ lymphocytes clearly involves only resting cells (i.e., CD25-CD4+ cells) while CD25highCD4+ and CD25lowCD4+ cells are "resistant" to this action of the drug. Determining whether this "resistance" is conditioned by the presence CD25 or whether the observed correlation is accidental requires further research. The available literature does not contain any precisely comparable data, but there have been reports about the effect of MEL on the levels of some cytokines in rat serum [21] or culturing medium of mouse splenocytes [20], or the mRNA expression of these cytokines [14]. Maeda et al. [14] did not observe any effect of MEL on IFN-γ mRNA expression in cultured PBMCs from calves. On the other hand, Oliveira et al. [21] found that administration of MEL in rats infected with Trypanosoma cruzi significantly increases the serum level of IFN-γ whereas splenocytes from mice infected with the same parasite produce significantly less of the cytokine when cultured with the drug [20]. These findings show that data demonstrating the effect of MEL on IFN-γ production are contradictory while our observations indicating that MEL may increase production of the cytokine are not unique. The most probable explanation for our result is that IFN-γ production depends on COX-2 activity and PGE2 production. Sharma et al. [23] demonstrated that COX-2 inhibition strongly induces IFN-γ production. Splenocytes from COX-2 inhibitor-treated mice have an enhanced capacity to secrete IFN-γ. Moreover, it has been demonstrated [10] that PGE2 inhibits the production of IFN-γ by CD4+ cells from human cord blood. It was not observed in the present study that MEL significantly changed the percentage of IL-10+ or TGF-β+ cells in the subpopulations being evaluated. We hypothesized that MEL might reduce IL-10 production because it has been previously demonstrated that COX-2-mediated PGE2 enhances the production of IL-10, and that COX-2- produced PGE2 might be essential for the reciprocal regulation of IL-10 and IL-12 production because it enhances IL-10 levels and spontaneously inhibits IL-12 production in LPS-treated bone marrow-derived dendritic cells (BM-DC) [7]. Moreover, there have been reports indicating that PGE2 can also regulate IL-10 synthesis in lymphocytes. MEL has been shown to reduce production of PGE2 by mouse splenocytes obtained from animals infected with Paracoccidioides brasiliensis [19] or Trypanosoma cruzi [20]. COX-2 is also associated with TGF-β production because its inhibition decreases TGF-β expression in some types of cultured cells [4]. However, these results clearly indicate that the production of IL-10 and TGF-β in bovine CD4+ lymphocytes does not depend on PGE2, or that the doses of MEL used do not inhibit COX-2 activity to an extent sufficient for reducing cytokine production.
In summary, our results demonstrated that MEL did not induce immunosuppression via the depletion of CD4+ cells or decreased production of IFN-γ and increased IL-10 or TGF-β synthesis by these cells. The ability to generate Foxp3+CD25highCD4+ cells indicates that the potential for such an effect exists. However, due to the small scale observed one should be cautious in attributing any clinical importance to this finding until appropriate studies have been conducted. The results obtained showed that with respect to the parameters being evaluated, meloxicam seems a relatively safe anti-inflammatory drug to be used in infectious diseases in cattle. Moreover, the drug seems to be able to induce IFN-γ production by CD4+ lymphocytes, which can be a positive attribute in patients who suffer from infections caused by intracellular pathogens.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download