Abstract
In the event of an infectious disease outbreak in cattle, carcasses must be disposed of in a rapid and contained manner. This brief communication details injection of a barbiturate to euthanize cattle inoculated with Escherichia coli O157:H7 followed by carcass composting in a manner that prevents the spread of infectious agents.
Intensification and globalization of livestock production have increased the potential for epizootic or pandemic disease outbreaks along with possible bioterrorism incidences [1,3]. An emergency situation can arise when a herd is infected with a pathogen that poses a threat to human and animal health as well as the livestock industry [5]. Infectious agents meriting emergency control measures in cattle include bacteria (Bacillus anthracis), viruses (Aphtae epizooticae), and prion proteins (transmissible spongiform encephalopathies) [7]. Since these infections preclude livestock from entering the food and feed chains, disposal of infected carcasses should be performed on-site or in close proximity. In the event of a pathogenic outbreak, a rapid and decisive eradication plan must be implemented (Fig. 1).
The principle concerns of culling animals during an emergency situation include: i) disease spread, ii) animal welfare prior to and during euthanasia, and iii) personnel safety [5,7]. There are several methods available for euthanizing animals in an outbreak situation depending on the species [5,7]. The method used should result in immediate loss of consciousness due to sedation or stunning followed quickly by death. For large animals such as cattle, these techniques include the use of firearms, a penetrating captive bolt followed by exsanguination or pithing, and barbiturates [2].
When selecting a methodology to eliminate infected animals, the nature of the pathogen must first be considered including the infectious dose, modes of transmission, and environmental persistence. For pathogens with prolonged environmental persistence (e.g., prions or endospores), contamination of the environment must be minimized. Pathogens potentially present in blood or other bodily fluids may necessitate non-invasive killing strategies to limit exposure to personnel and the environment. The size, maturity, and number of animals to be sacrificed as well as space, technology, and resources required must all be considered. Furthermore, climatic conditions can affect the feasibility and ease of implementing euthanasia strategies. Importantly, animals should be euthanized humanely with minimal pain and distress in a manner that is safe for workers and minimizes negative perception by the public.
Other important considerations are the transport and disposal of carcasses and infected materials (e.g., bedding material, manure, and soil) after euthanasia (Fig. 1). Transportation is influenced by infrastructure, technology, climatic conditions, and pathogenic potential of the infectious agent. Short distances and rapid transportation are ideal. Large numbers of carcasses and/or transportation logistics may necessitate the use of equipment that is liquid-tight, simple to disinfect, and temperature controlled to prevent carcasses from decomposing or freezing.
Carcass disposal options will vary according to the animal and pathogen, but may include incineration (open pyres or enclosed facilities), rendering, burial, or composting [7]. The method of euthanasia chosen should complement the disposal strategy with distance between the knockdown and disposal sites being a critical factor. Ideally, euthanasia and carcass disposal should both be performed on-site.
In Canada, there are limited guidelines for large-scale, on-site euthanasia of infected animals [6]. The following novel case study arose from the need to euthanize and dispose a cattle herd experimentally infected with Escherichia (E.) coli O157:H7, which prevented the animals from entering the food chain. The carcasses were disposed of in compost structures previously constructed on-site as outlined by Reuter et al. [4]. The lack of published euthanasia procedures encouraged us to document the decision-making process and procedures used to depopulate the cattle in this study. To the best of our knowledge, this is the first documented case study of its kind.
Crossbred steers (n = 32) inoculated with E. coli O157:H7 three months earlier were euthanized prior to disposing the carcasses by composting. These cattle were confirmed to be infected with E. coli O157:H7 by fecal monitoring using immunomagnetic separation with anti-E. coli O157 Dynabeads (Invitrogen, USA) according to manufacturer's instructions, and plating on Sorbitol MacConkey agar with 2.5 mg/L potassium tellurite, 0.05 mg/L cefixime and 50 mg/L nalidixic acid (Dalynn Biologicals, Canada). All steers were handled in accordance with the Canadian Council of Animal Care Guidelines. The animals were identified by a unique ear tag and weighed an average of 617 ± 33 kg.
Cattle to be euthanized were sequentially herded one at a time from the feedlot where they were housed, down a fenced corridor, and into a mobile cattle squeeze (Hi Hogg Farm and Ranch Equipment, Canada; Fig. 2). The squeeze was secured to the ground with steel rods and surrounded by a fence to ensure secondary containment of infected cattle if they were to escape. The ground under and around the squeeze was covered with a layer of sawdust (approximately 5 cm deep) to mitigate contamination of the soil with potentially infectious materials including blood, manure, or other fluids. Once restrained in the squeeze, the cattle were given an intramuscular (IM) injection of 100 mg xylazine HCl (1 mL of Xylamax; Bimeda-MTC Animal Health, Canada). Xylazine was administered IM instead of intravenously because this method facilitated sedation of the cattle with minimal disturbance. Approximately 2 min after the xylazine was given, a halter was placed around the head of the cattle and the rope was secured to a hook on the squeeze, thereby securing the head to one side and exposing the neck. The vena jugularis externa was readily identified, and a 5-cm, 14 G needle (Becton Dickinson, USA) connected to 20 cm of polyethylene intramedic catheter tubing (I.D. 1.67 mm; Becton Dickinson, USA) was inserted into the vein. Proper needle placement was confirmed by observing a reflux flow of blood into the tubing (without blood spilling onto the ground). A syringe was attached to the catheter and Euthanyl Forte (540 mg/mL sodium pentobarbital; Bimeda-MTC Animal Health, Canada) was injected at a minimum dosage of 113 mg sodium pentobarbital/kg of body weight, slightly exceeding the manufacturer's recommended lethal dose of 108 mg/kg. Cattle were considered deceased after loss of pupilliary reflex and brain death due to cardiac and/or respiratory arrest [2]. The deceased cattle were pulled out of the squeeze by their neck using a steel chain attached to a Thomas 135 Skid Steer (Thomas Equipment, Canada). Once removed from the squeeze, each carcass was lifted, carried, and transported 100 m to a composting site using a Komatsu 120 Pay Loader (Komatsu, Japan) with an attached fork.
After the final steer was sacrificed, the area around the chute was cleaned by collecting sawdust from the ground and placing it in the compost structure. All disposable items were collected in biohazard bags (Thermo Fisher Scientific, USA) for subsequent autoclaving. All machinery and tools were decontaminated with a broad-spectrum, multi-purpose disinfectant (Virkon; DuPont, USA) using a backpack sprayer according to the manufacturer's instructions.
In the present study, advance knowledge of the need to dispose of the cattle herd gave us an advantage over a "real" infectious outbreak for facilitating the planning and preparation of our euthanasia strategy and disposal sites well in advance. This made the process more efficient than what may be possible during a true emergency response. Based on the epidemiology of E. coli O157:H7, we determined that a non-invasive euthanasia strategy would be optimal to ensure personnel safety, minimize environmental contamination, and complement carcass disposal via composting. The effectiveness of composting for carcass disposal and E. coli O157:H7 elimination was previously demonstrated by our research group [4]. Invasive euthanasia methods such as the use of a captive stunning bolt were undesirable since the potential need for exsanguination or pithing to ensure animal death would have caused substantial environmental contamination, and in some cases of infectious outbreaks could present substantial risk of personnel exposure to infected blood. Using firearms was also undesirable due to the risk to personnel injury by ricocheting bullets in a partially enclosed area, distress to other animals caused by the noise of firearm discharge, and the potential for negative public perception. Elimination of these euthanasia methods left barbiturate injection as the most desirable option.
Out of the 32 cattle euthanized, only six were uncooperative or difficult to handle prior to sedation. However, it should be noted that these cattle as a group were accustomed to being handled and placed into the squeeze given that they had been routinely restrained during a larger challenge experiment conducted with E. coli O157:H7. Considerable emphasis should be placed on having experienced personnel working with the cattle, and making a concerted effort to handle the animals in a quiet manner to minimize disruptions. In that regard, there is at least an anecdotal belief that cattle which become very agitated prior to sedation may be unpredictable and dangerous. Furthermore, agitated cattle are less sensitive to barbiturates, making them much more difficult to handle, increasing the risk of injury to personnel, and causing more psychological distress to the personnel and animals involved.
Time intervals of our experimental procedure (Fig. 3) were as follows: entering the squeeze to administration of the sedative (< 1 to 2 min), administration of the sedative to Euthanyl injection (1 to 3 min, average of 2.2 min), and administration of Euthanyl to confirmation of death (2 to 27 min, average of 5.2 min). For the interval between Euthanyl administration and death, times for two animals (11 and 27 min) were considered outliers. Both cattle were combative, and resisted handling and the injections. It is possible that the agitated state of these cattle delayed the action of the barbiturate. Furthermore, despite appropriate restraint the movement of these cattle in the squeeze (Fig. 4) may have caused a portion of the barbiturate to be injected into perivascular tissues. A potential way to shorten the interval between animal restraint and loss of consciousness would be to administer xylazine intravenously, resulting in a more rapid onset of sedation. Regardless, it is noteworthy that there were no indications that any of the cattle experienced undue pain or distress caused by the procedure. The interval between confirmation of death and removal of the carcass from the squeeze ranged from < 1 to 2 min (average of 1.2 min). Although the time required for transporting carcasses to the disposal site (100 m away), placement, and covering was not recorded, all 32 cattle were euthanized and sealed inside the biosecure compost structure within a span of 11 h (Figs. 3 and 4). Due to the nature of the personal protective equipment (PPE) and potential for personnel contamination, several restrictions need to be considered during an emergency situation. When including preparations prior to culling and post-operation clean-up, the procedure was completed in two shifts of approximately 5 h each. During each shift, the disposal team had no access to water, food, bathroom facilities, or communication devices without a required change of PPE and personal decontamination procedures. During the second shift (afternoon), workers experienced problems with dust due to increased wind as well as increasing sun intensity and temperatures of 26℃ that reduced both visibility (fogged glasses and face shields) and increased worker discomfort. One team member outside the working area was responsible for documenting the entire procedure, maintaining external communication, and ordering additional materials as required. This member had no direct contact with the team or equipment area but was located within a range suitable for verbal communication. Although the reported conditions caused some worker discomfort, more extreme weather conditions such as substantial precipitation, wind speeds above 57 km/h, or extreme temperatures would have to be considered for equipment functionality if worker wellbeing is to be assured during the containment and disposal of livestock.
Barbiturate injection was an effective method for euthanizing cattle prior to their disposal via composting. The procedure was humane, biosecure, and efficient for culling a herd of 32 cattle previously inoculated with E. coli O157:H7. Although this technique could not be feasibly applied to very large numbers of cattle without increasing the number of personnel and animal restraint systems, it would be an effective method for dealing with small herds in the event of a disease outbreak. The direct costs of euthanasia (including drugs, disposables, and PPE) was approximately CAN $ 60 per animal.
Globalization, increased housing density of livestock, and intensification of livestock production systems have created an increased potential for infectious agents to cause epizootic or pandemic disease outbreaks. To prevent and/or control outbreaks, immediate emergency euthanasia of livestock at the risk of infecting humans and other animals may be necessary. Regardless, it is imperative that the euthanasia of livestock be conducted with consideration for animal welfare. The options for effective and humane euthanasia of large domestic livestock are limited by the body size and number of livestock requiring disposal. Additional considerations include climate, infrastructure, mode and distance of carcass transportation, legislation, expertise, manpower, available funds, and potential emotional attachments. The complexity of infectious outbreak situations and emergency strategies to eliminate outbreaks can make it difficult to implement a standard operating protocol. Optimal euthanasia techniques require the consideration of possible on-site limitations within an emergency strategic plan. Preparatory operations should include emergency training of staff members, an adequate supply of personal safety equipment, and the establishment of step-by-step action and disposal plans.
Figures and Tables
Fig. 1
Flow diagram of the steps necessary for eradicating infectious animal diseases with epizootic or pandemic potential.
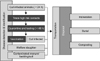
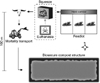
Fig. 2
Diagram of the site for the euthanasia and disposal of cattle previously inoculated with E. coli O157:H7 in a biosecure compost structure. Cattle were individually herded from their feedlot pen into a mobile squeeze apparatus where they were euthanized. The carcass was then transported from the euthanasia site to the compost structure along a gravel road.
Acknowledgments
The authors thank R. Wilde, M. Anderson, B. Baker, R. Barbieri, H. Daniels, B. Jensen, and W. Smart for technical assistance. This project was funded by grants from the Agriculture and Agri-Food Canada peer-reviewed project program and the Beef Cluster program of the Beef Cattle Research Council.
References
2. Galvin JW, Blokhuis H, Chimbombi MC, Jong D, Wotton S. Killing of animals for disease control purposes. Rev Sci Tech. 2005. 24:711–722.
3. Gerber P, Chilonda P, Franceschini G, Menzi H. Geographical determinants and environmental implications of livestock production intensification in Asia. Bioresour Technol. 2005. 96:263–276.

4. Reuter T, Xu W, Alexander TW, Gilroyed BH, Inglis GD, Larney FJ, Stanford K, McAllister TA. Biocontained carcass composting for control of infectious disease outbreak in livestock. J Vis Exp. 2010. 39:e1946.

5. The European Union. Council Regulation (EC) No 1099/2009 of 24 September 2009 on the protection of animals at the time of killing. Off J Eur Union. 2009. 52:1–30.
6. Whiting TL. Foreign animal disease outbreaks, the animal welfare implications for Canada: risks apparent from international experience. Can Vet J. 2003. 44:805–815.
7. World Organisation for Animal Health. Terrestrial Animal Health Code. 2006. 15th ed. Paris: OIE;475–496.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download