Abstract
The follicle-associated epithelium (FAE) of Peyer's patches (PPs) contains M cells that are important for reducing mucosal immune responses by transporting antigens into the underlying lymphoid tissue. We generated a monoclonal antibody (C6) that reacted with the FAE of calf ileal PPs, and analyzed the characteristics of C6 using immunohistochemistry and Western blotting. FAE of the ileal PP was stained with C6 during both late fetal developmental and postnatal stages. Neither the villous epithelial cell nor intestinal crypt basal cells were stained at any developmental stage. During the prenatal stages, FAE of the jejunal PP was C6-negative. However, a few C6-positive cells were distributed diffusely in some FAE of the jejunal PPs during the postnatal stages. The protein molecular weight of the antigen recognized by C6 was approximately 45 kDa. These data show that C6 is useful for identifying the FAE in ileal PPs and further suggest that differentiation of the FAE in these areas is independent of external antigens.
There are two types of Peyer's patches (PPs) in calf intestine: ileal and jejunal [19]. These tissues consist of lymphatic follicles, interfollicular T cell areas, and dome regions [4]. The dome regions are covered by follicle-associated epithelium (FAE) containing "microfold" or "membranous" (M) cells which differ from intestinal epithelial cells morphologically and functionally [18,19].
The jejunal PP is thought to be a secondary lymphoid tissue similar to other mammalian PPs, and the FAE consists of M cells scattered among intestinal epithelial cells. In contrast, the ileal PP is thought to be composed of primary lymphoid tissue containing B cells and most areas of the FAE are covered by M cells [19,22]. M cells are important for initiating mucosal immune responses by transporting antigens into the underlying lymphoid tissues. These cells can also form entry points for pathogens such as invasive Salmonella Typhimurium which selectively penetrates and destroys M cells in intestinal PPs [15]. M cells may also deliver human immunodeficiency virus type 1 to target cells in mucosal lymphoid tissues [1]. In calves and lambs, prion protein has been found in FAE of the ileal PP, suggesting that M cells are important for uptake and therefore contribute to oral infection susceptibility [10,11,27].
Ulex europaeus agglutinin 1 (UEA-1) is a specific marker of mouse M cells [6]. CCL20 is a chemokine expressed in both mouse and human FAE [2,26,28]. Gebert et al. [8,9] have also shown that cytokeratin 18 and vimentin are useful markers of porcine and rabbit M cells, respectively. However, a bovine M cell-specific marker has not yet been identified. In addition, ontogeny and differentiation mechanisms of M cells are not fully understood and need further detailed investigation. In the present study, a monoclonal antibody specific for the FAE of calf ileal PPs was generated during ontogeny and characterized using immunohistochemistry, scanning electron microscopy (SEM), and Western blotting.
Japanese black calves (fetuses 5~9 months old, calves aged 2~10 days, 1~3 months, and 8 months; n = 3 for each developmental stage) were obtained from a local farm and slaughterhouses in the Miyazaki Prefecture (Japan). Fetal age was estimated by crown-to-rump measurements. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Miyazaki (Japan). The small intestines including ileal and jejunal PPs were collected for immunohistochemistry, SEM, and Western blotting. For immunohistochemistry, the specimens were mounted in an optimal cutting temperature embedding compound (OCT compound; Sakura Finetek Japan, Japan) on Cryomold (Sakura Finetek Japan, Japan), frozen on dry ice, and then stored at -80℃ until analysis.
Culture supernatant of a C6 hybridoma (C6) was generated as described previously [23] and used as a primary antibody. Briefly, mice were repeatedly immunized with a mixture of ovine monocyte-derived dendritic cells (DCs) generated in vitro and a population of ovine afferent intestinal lymphatic cells which contained approximately 15% mature DCs. Mouse antibody responses of CD11c+ ovine afferent lymphatic cells to DC surface antigens were assessed by a FACS Caliber flow cytometry (Applied Biosystems, USA) to identify the animal with the greatest response which then was subjected to a final boost consisting of only DCs. Screening of the hybridoma supernatants for DC binding was also performed by flow cytometry using afferent intestinal lymph cells [23].
Cryostat sections were stained with C6 using an indirect immunoperoxidase technique previously described by Yasuda et al. [30]. Briefly, sections (7- to 10-µm thick) were air dried on slides (Matsunami Glass Ind., Japan) and fixed with ice-cold acetone for 10 min. To block nonspecific binding, the sections were rehydrated in phosphate-buffered saline (PBS) and incubated with 10% normal goat serum (Vector Laboratories, USA) in PBS for 30 min at room temperature. The sections were incubated with C6 (culture supernatant of hybridoma) for 60 min at room temperature and washed three times with PBS. Next, the slides were incubated with biotin labeled horse anti mouse IgG (H+L) as a secondary antibody for 30 min at room temperature (Vector Laboratories, USA) absorbed with acetone powder of calf jejunal and ileal PPs. Endogenous peroxidase activity was then quenched with 0.3% H2O2 in methanol for 30 min at room temperature followed by incubation with ABC complex (Vector Laboratories, USA) for 15 min at room temperature. After the sections were rinsed three times in PBS, the reactions were visualized with metal-enhanced diaminobenzidine (Thermo Fisher Scientific, USA). Immunohistochemical staining was performed at room temperature in a incubation chamber (Cosmo-Bio, Japan). Control staining was simultaneously performed in which the primary antibody was replaced with normal mouse IgM (1,000 times dilution; Santa Cruz Biotechnology, USA). No positive staining was found in the control slides (data not shown).
Tissues were fixed in 2.5% glutaraldehyde (Nacalai Tesque, Japan), washed with 0.1 M phosphate buffer (PB; pH 7.4), and then further fixed with 1% osmium tetroxide (Merck, Germany) in 0.1 M PB. After dehydration with a series of graded ethanol solutions and substitution with isoamyl acetate, the specimens were dried in a critical point dryer (Type HCP-2; Hitachi, Japan) and coated with gold by ion sputter (E-1030; Hitachi, Japan). The specimens were then examined by SEM (S-4100; Hitachi, Japan).
Cells from calf ileal PPs were lysed in lysis buffer [TNE buffer (10 mM Tris-Cl, pH 7.8; 0.01% NaN3, 150 mM NaCl, 1 mM EDTA, and 1% NP-40), 1 mM phenylmethanesulfonylfluoride or phenylmethylsulfonyl fluoride, 0.3% aprotinin (Sigma-Aldrich, USA), and 0.1% phosphatase inhibitor cocktail 2 (Sigma-Aldrich, USA)], vortexed, and centrifuged at 15,000 × g for 20 min at 4℃. The proteins were heated to 96℃ for 5 min in Laemmli sample buffer (Bio-Rad Laboratories, USA), resolved on a 3~10% polyacrylamide gel in running buffer (Tris/glycine/SDS buffer; Bio-Rad Laboratories, USA), and transferred to polyvinylidene difluoride (PVDF) membranes (Immuno-Blot PVDF Membrane; Bio-Rad Laboratories, USA). The membranes were blocked overnight in blocking buffer (GE Healthcare Bio-Sciences, UK) at 4℃ and then incubated with C6 (culture supernatant of hybridoma) for 60 min at room temperature. The membranes were washed by 0.01% teen20-PBS, and incubated with secondary antibodies (horseradish peroxidase-conjugated anti-mouse IgM heavy chain, 100,000 times dilution; Millipore, USA) for 30 min at room temperature. Next, the membranes were again washed by 0.01% Tween 20-PBS and incubated with detection reagent (GE Healthcare Bio-Sciences, UK) for 5 min. A light capture system and CSanalyzer (ATTO, Japan) were used to detect antibody binding. Reactivity of C6 to the ovine antigens was also assessed using a standard procedure in which proteins from afferent intestinal lymph cells were lysed in 1% of NP-40, resolved on a 10% polyacrylamide gel, and visualized with a 3-amino-9-ethylcarbazole staining kit (Sigma-Aldrich, USA).
A summary of the C6+ cell distribution in calf PPs during ontogeny is shown in Table 1. During the middle fetal stage (Fig. 1A; fetal 5 months), no ileal PP lymphoid tissue formation or C6-positive reactions in the ileum were detected. During the late fetal stages (Figs. 1B and C; fetal 7 and 9 months, respectively), ileal PP formation was observed and C6+ cells were distributed in the FAE but not among the villous epithelial cells. Many cells covering the FAE were C6-positive and a small subset was C6-negative. Jejunal PP formation was also found during fetal development, but neither the FAE nor villous epithelial cells reacted with C6 (Fig. 1D; fetal 9 months).
At the postnatal stage (2~10 days), almost the entire FAE in the ileal PP was C6-positive while the villous epithelium was negative (Fig. 2A). The epithelial cells changed from C6-negative to C6-positive along the crypt-to-dome axis (Fig. 2A) while all epithelial cells were negative along the crypt-to-villus axis (Fig. 2A). At the height of ileal PP development (around 1~3 months) and the start of involution (8 months), all epithelial areas in the FAE were C6-positive with a staining pattern similar to that found in PPs from the earlier postnatal stage (Fig. 2A). In addition, the cytoplasm as well as the membranes of cells in the FAE were C6-positive, but not in the villous epithelium (Fig. 2C). During the postnatal stages, very few positive cells were distributed in some FAE of the jejunal PP (Figs. 2B and D). The smooth muscles layer and smooth muscular fibers in the lamina propria were also C6-positive (arrows in Fig. 2). Hassall's corpuscle in the thymus medulla and epithelial layers of the palatine and pharyngeal tonsil were also C6-positive (Figs. 3A~C). In addition, reticular cells in the lymphatic follicle of the mesenteric lymph nodes, ileal PP lymphatic follicles, and the spleen were C6-positive (Figs. 3D~F). In these tissues, cells with the morphologies of DCs and macrophages also reacted with C6.
Morphological differences between M cells in FAE of ileal and jejunal PPs were observed by SEM. The villous epithelium of jejunal PPs had regular and dense microvilli during the postnatal stage (at 3 days) while M cells with irregular microvilli were sparsely scattered throughout the FAE (Figs. 4A and B). M cells with irregular microvilli covered the entire FAE of the ileal PP (Figs. 4C and D). In addition, cells similar to M cells in FAE of the ileal PP at the prenatal stage were also observed (data not shown). The monoclonal C6 antibody reacted with a bovine ileal PP protein as well as an ovine protein approximately 45 kDa in size expressed in migrating afferent intestinal lymph cells as shown by Western blot analysis (Fig. 5).
FAE overlying lymphatic follicles of PPs in many domestic animals is a key player in the initiation of mucosal immune responses. Intermediate filaments such as vimentin, cytokeratin 8, and cytokeratin 18 have been identified as M cell markers in rabbit, rat, and pig, respectively [8,9,13,25]. In addition, lectin UEA-1 and peanut agglutinin are useful M cell markers in mouse and rabbit, respectively [6,14]. The chemokines CCL20 and CCL9 are expressed in mouse and human FAE. CCL20 recruits immature DCs to the FAE for antigen capturing and the induction of acquired immunity [2,26,28,31]. In calves, M cells can be distinguished from the villous epithelium by the different actin and villin staining patterns although these proteins are not exclusively expressed on the FAE [16].
In the present study, we described the C6 monoclonal antibody specific for a calf FAE antigen. We then analyzed staining patterns in the FAE of ileal and jejunal PPs during ontogeny. During the middle fetal developmental stage, jejunal but not ileal PP formation is observed [29]. At the late fetal developmental stage, C6+ cells were found in the FAE as described in this study. Previous histological observations indicated that the FAE is not developed in the ileum of calf fetuses at 73~150 days of gestation, but the FAE are distinguishable in fetuses 7~9 months old [3,5]. However, FAE in the jejunal PP did not react with C6 during the prenatal stages. According to the distribution of C6+ cells and the morphology of cells covering the FAE, M cells might therefore arise from epithelial cells in the ileal PP during the late fetal stage. In addition, the data suggest that FAE development in ileal PPs is different from that in jejunal PPs.
At the postnatal stages, C6+ cells covered all areas in the FAE of ileal PPs. In addition, very few C6+ cells were found in some FAE of the jejunal PP after birth. M cells were scattered throughout the FAE of jejunal PPs. Therefore, C6+ cell localization closely mirrored the distribution of M cells observed by SEM. However, additional studies are required to determine whether or not C6+ cells in the FAE of jejunal PPs are indeed M cells. Some DC and macrophages were also stained by C6. Furthermore, C6+ cells were found in the epithelium of Hassall's corpuscles of the thymus medulla and tonsils. We therefore speculate that the antigen recognized by C6 is a cellular protein that is involved in cell motility of antigen presenting, M, and epithelial cells. This hypothesis, however, requires further investigation.
Whether or not M cells are derived from epithelial cells is still unclear. It has been reported that human Caco-2 cells are converted into M cell by coming into contact with PP lymphocytes in vitro [17]. In addition, the absence of mature lymphocytes does not prevent the formation of M cells in the small intestine of Rag-1-/- mice in vivo [7]. M cells also develop in mouse intestinal villi [12]. However, the development of M cells in the villi is independent of the presence of lymphocytes while M cell development in the FAE is dependent on B cells [21]. This indicates that the signals for M cell differentiation vary between different anatomical compartments. In addition, previous studies of B cell-depleted fetal sheep showed that ileal PPs contain differentiated FAE and reticular cells [20,24]. Therefore, we speculate that development of the FAE in primary lymphoid organs is distinct from that of FAE in secondary lymphoid organs.
In conclusion, the results of our study showed that C6 is a useful marker of FAE in calf ileal PPs. C6 can also be potentially used for analyzing the functions and ontogeny of calf M cells in the FAE of ileal PPs.
Figures and Tables
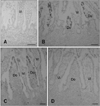 | Fig. 1C6+ cell distribution in the FAE of ileal and jejunal Peyer's patches (PPs) during the prenatal stages. (A) No ileal PP lymphoid tissue formation was observed in a 5-month old fetus. All cells were C6-negative. (B) In a 7-month old fetus, ileal PP formation was observed. A subset of cells in the FAE but not the villus epithelium was C6 positive. (C) In a 9-month old fetus, C6+ cells were found in FAE of the ileal PP but the villous epithelial cells were C6-negative. (D) No C6-positive cells were found in FAE of the jejunal PP or villus epithelium of the 7~9-month old fetuses. Do: dome region and Vi: villus. Scale bars = 50 µm. |
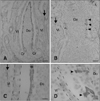 | Fig. 2C6+ cell distribution in the FAE of ileal and jejunal PPs during the postnatal stages. (A and C) FAE in the ileal PP was stained by C6 during the postnatal stages. In the ileal PP, the epithelial cells converted from negative to C6-positive along the crypt-to-dome axis but all epithelial cells were negative along the crypt-to-villus axis. The asterisk indicates C6+ FAE in the ileal PP dome region. The broken lines represent the outline of villous epithelial cells which were C6-negative. (B and D) Very few C6+ cells were found in some FAE of the jejunal PP (arrowhead). The broken lines represent the outline of villous epithelial cells. Arrows indicate the smooth muscular fibers in the lamina propria. Do: dome region, Vi: villus, and Cr: crypt. Scale bars = 50 µm (A and B) and 10 µm (C and D). |
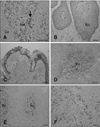 | Fig. 3The distribution of C6+ cells in other lymphoid tissues. (A) Hassall's corpuscle (arrow) in the thymus medulla was C6-positive. Epithelial layers of the palatine tonsil (B; stratified squamous epithelium) and pharyngeal tonsil (C; pseudostratified epithelium) were also C6-positive. Reticular cells in lymphatic follicles of the mesenteric lymph node (D), ileal PP lymphatic follicles (E), and the spleen (F) were C6-positive. Me: thymus medulla, Co: thymus cortex, Ep: epithelium, and Fo: lymphatic follicle. Scale bars = 50 µm. |
 | Fig. 4Scanning electron micrograph (SEM) of the FAE in ileal and jejunal PPs. (A) The dome region (arrowhead) of the jejunal PP was surrounded by villi. (B) M cells with irregular microvilli were sparsely scattered in FAE of the jejunal PP. (C) Many dome regions of the ileal PP were observed. (D) The majority of cells in the FAE were M cells with irregular and sparse microvilli. Scale bars = 50 µm (A), 5 µm (B), 70 µm (C), and 3 µm (D). |
Acknowledgments
The authors wish to thank the staff of the Miyakonojo Meat Inspection Center, Miyazaki Prefecture, Japan, for kindly providing the fetal calves used for this study. This work was supported by a Grant-in-Aid for Scientific Research (No. 23405042) from the Ministry of Education, Science, Sport and Culture, Japan; and by OVITA, New Zealand.
References
1. Amerongen HM, Weltzin R, Farnet CM, Michetti P, Haseltine WA, Neutra MR. Transepithelial transport of HIV-1 by intestinal M cells: a mechanism for transmission of AIDS. J Acquir Immune Defic Syndr. 1991. 4:760–765.
2. Anderle P, Rumbo M, Sierro F, Mansourian R, Michetti P, Roberts MA, Kraehenbuhl JP. Novel markers of the human follicle-associated epithelium identified by genomic profiling and microdissection. Gastroenterology. 2005. 129:321–327.

3. Asari M, Kano Y, Wakui S, Nishita T, Matsushita H, Oshige H. The ultrastructure of bovine ileal follicle-associated epithelial (FAE) cells during the perinatal period. J Anat. 1989. 163:7–16.
4. Azzali G. Structure, lymphatic vascularization and lymphocyte migration in mucosa-associated lymphoid tissue. Immunol Rev. 2003. 195:178–189.

5. Beyaz F, Aşti RN. Development of ileal Peyer's patches and follicle associated epithelium in bovine foetuses. Anat Histol Embryol. 2004. 33:172–179.

6. Clark MA, Jepson MA, Simmons NL, Booth TA, Hirst BH. Differential expression of lectin-binding sites defines mouse intestinal M-cells. J Histochem Cytochem. 1993. 41:1679–1687.

7. Debard N, Sierro F, Browning J, Kraehenbuhl JP. Effect of mature lymphocytes and lymphotoxin on the development of the follicle-associated epithelium and M cells in mouse Peyer's patches. Gastroenterology. 2001. 120:1173–1182.

8. Gebert A, Hach G, Bartels H. Co-localization of vimentin and cytokeratins in M-cells of rabbit gut-associated lymphoid tissue (GALT). Cell Tissue Res. 1992. 269:331–340.

9. Gebert A, Rothkötter HJ, Pabst R. Cytokeratin 18 is an M-cell marker in porcine Peyer's patches. Cell Tissue Res. 1994. 276:213–221.

10. Heggebø R, Press CM, Gunnes G, Lie KI, Tranulis MA, Ulvund M, Groschup MH, Landsverk T. Distribution of prion protein in the ileal Peyer's patch of scrapie-free lambs and lambs naturally and experimentally exposed to the scrapie agent. J Gen Virol. 2000. 81:2327–2337.

11. Heppner FL, Christ AD, Klein MA, Prinz M, Fried M, Kraehenbuhl JP, Aguzzi A. Transepithelial prion transport by M cells. Nat Med. 2001. 7:976–977.

12. Jang MH, Kweon MN, Iwatani K, Yamamoto M, Terahara K, Sasakawa C, Suzuki T, Nochi T, Yokota Y, Rennert PD, Hiroi T, Tamagawa H, Iijima H, Kunisawa J, Yuki Y, Kiyono H. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc Natl Acad Sci USA. 2004. 101:6110–6115.

13. Jepson MA, Mason CM, Bennett MK, Simmons NL, Hirst BH. Co-expression of vimentin and cytokeratins in M cells of rabbit intestinal lymphoid follicle-associated epithelium. Histochem J. 1992. 24:33–39.

14. Jepson MA, Simmons NL, Hirst GL, Hirst BH. Identification of M cells and their distribution in rabbit intestinal Peyer's patches and appendix. Cell Tissue Res. 1993. 273:127–136.

15. Jones BD, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J Exp Med. 1994. 180:15–23.

16. Kanaya T, Aso H, Miyazawa K, Kido T, Minashima T, Watanabe K, Ohwada S, Kitazawa H, Rose MT, Yamaguchi T. Staining patterns for actin and villin distinguish M cells in bovine follicle-associated epithelium. Res Vet Sci. 2007. 82:141–149.

17. Kernéis S, Bogdanova A, Kraehenbuhl JP, Pringault E. Conversion by Peyer's patch lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997. 277:949–952.

18. Landsverk T. Phagocytosis and transcytosis by the follicle-associated epithelium of the ileal Peyer's patch in calves. Immunol Cell Biol. 1988. 66(Pt 4):261–268.

19. Landsverk T. The follicle-associated epithelium of the ileal Peyer's patch in ruminants is distinguished by its shedding of 50 nm particles. Immunol Cell Biol. 1987. 65(Pt 3):251–261.

20. Lie KI, Press CM, McCullagh P, McClure SJ, Landsverk T. Differentiation of the follicle-associated epithelium in ileal Peyer's patch and production of 50-nm particles are maintained in B-cell-depleted fetal sheep. Cell Tissue Res. 2005. 319:395–404.

21. Mach J, Hshieh T, Hsieh D, Grubbs N, Chervonsky A. Development of intestinal M cells. Immunol Rev. 2005. 206:177–189.

22. Parsons KR, Bland AP, Hall GA. Follicle associated epithelium of the gut associated lymphoid tissue of cattle. Vet Pathol. 1991. 28:22–29.

23. Pernthaner A, Cole SA, Gatehouse T, Hein WR. Phenotypic diversity of antigen-presenting cells in ovine-afferent intestinal lymph. Arch Med Res. 2002. 33:405–412.

24. Press CM, Reynolds JD, McClure SJ, Landsverk T. Development of accessory cells in B-cell compartments is retarded in B-cell-depleted fetal sheep. Dev Immunol. 1998. 6:223–231.

25. Rautenberg K, Cichon C, Heyer G, Demel M, Schmidt MA. Immunocytochemical characterization of the follicle-associated epithelium of Peyer's patches: anti-cytokeratin 8 antibody (clone 4.1.18) as a molecular marker for rat M cells. Eur J Cell Biol. 1996. 71:363–370.
26. Rumbo M, Sierro F, Debard N, Kraehenbuhl JP, Finke D. Lymphotoxin β receptor signaling induces the chemokine CCL20 in intestinal epithelium. Gastroenterology. 2004. 127:213–223.

28. Tanaka Y, Imai T, Baba M, Ishikawa I, Uehira M, Nomiyama H, Yoshie O. Selective expression of liver and activation-regulated chemokine (LARC) in intestinal epithelium in mice and humans. Eur J Immunol. 1999. 29:633–642.

29. Yasuda M, Fujino M, Nasu T, Murakami T. Histological studies on the ontogeny of bovine gut-associated lymphoid tissue: appearance of T cells and development of IgG+ and IgA+ cells in lymphoid follicles. Dev Comp Immunol. 2004. 28:357–369.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download