Abstract
Chicken anemia virus (CAV) is an important viral pathogen that causes anemia and severe immunodeficiency syndrome in chickens worldwide. In this study, a potential diagnostic monoclonal antibody against the CAV VP1 protein was developed which can precisely recognize the CAV antigen for diagnostic and virus recovery purposes. The VP1 gene of CAV encoding the N-terminus-deleted VP1 protein, VP1Nd129, was cloned into an Escherichia (E.) coli expression vector. After isopropyl-β-D-thiogalactopyronoside induction, VP1Nd129 protein was shown to be successfully expressed in the E. coli. By performing an enzyme-linked immunoabsorbent assay using two coating antigens, purified VP1Nd129 and CAV-infected liver tissue lysate, E3 monoclonal antibody (mAb) was found to have higher reactivity against VP1 protein than the other positive clones according to the result of limiting dilution method from 64 clones. Using immunohistochemistry, the presence of the VP1-specific mAb, E3, was confirmed using CAV-infected liver and thymus tissues as positive-infected samples. Additionally, CAV particle purification was also performed using an immunoaffinity column containing E3 mAb. The monoclonal E3 mAb developed in this study will not only be very useful for detecting CAV infection and performing histopathology studies of infected chickens, but may also be used to purify CAV particles in the future.
Chicken anemia virus (CAV) is the sole member of the genus Gyrovirus of the Circoviridae family and causes severe anemia and chicken anemia disease, an immunosuppressive disorder [3,13,14]. Histopathological studies have shown that CAV infection leads to aplasia of the bone marrow [10,21]. This results in anemia and severe immunodeficiency syndrome due to the destruction of T lymphoid tissue [10,21].
The CAV genome consists of a circular single-stranded DNA genome of 2.3 kb encoding three viral proteins (VP): VP1, VP2 and VP3 [3,13,14]. VP1 is the sole structural protein of the CAV capsid. At a very late stage of the virus life cycle, the assembled virus particles created by VP1 spread into various other tissues and organs of chickens such as the thymus, spleen, and liver. Among these tissues and organs, liver tissue has been reported to have the highest accumulation of CAV virions [21]. Several methods have been developed to conventionally detect CAV infection such as serological tests for identifying CAV antibodies. Recently, immunohistochemistry (IHC) and immunofluorescence (IF) have been used as alternative methods for detecting CAV antigen [1,11,12,17,18]. For these methods to be successful, an excellent monoclonal antibody is essential. Thus, antigen preparation is a critical factor when producing these monoclonal antibodies. It has been reported that VP2 and VP3 have been used as target antigens to generate monoclonal antibodies for immunological characterization or for developing diagnostic enzyme-linked immunoabsorbent assay (ELISA) kits [5,21]. However, VP1 has rarely been used as the antigen for generating antibodies or for producing diagnostic kits. This is because problems with VP1 expression have been reported in several host cell systems [5,8,15,18].
Research on VP1 antigen preparation has generally been unsuccessful because of a failure to find a good recombinant protein expression system. The highly enriched span of arginine residues at the N-terminus of VP1 has been proposed to be cytotoxic in an Escherichia (E.) coli expression system [15]. Thus, there is a need to overcome the difficulties of VP1 antigen preparation. If successful, this would allow the generation of a monoclonal antibody against VP1 capsids that may potentially be used diagnostically for the clinical detection of CAV infections. Recently, our group has shown that the VP1Nd129 protein (amino acid residues 130~450 of VP1), from which the first 129 amino acid residues of the VP1 N-terminus have been deleted, can be used to successfully express large amounts of the protein in prokaryotic cells [8].
In this study, the truncated recombinant VP1Nd129 protein was used as antigen in immune BALB/c mice to develop and produce a number of monoclonal antibodies for immunological applications. One of these monoclonal antibodies, E3, was selected for evaluating the ability of the monoclonal antibody to recognize VP1 in clinical samples infected with CAV including liver and thymus tissue. In addition, an immunoaffinity column containing E3 mAb as a ligand for virus particle purification also was investigated herein. The results of our study will be very useful for developing immunological tools to detect CAV, identifying CAV infection, or for performing CAV histopathology studies in chickens.
CAV (CIA89) was provided by Professor Yi Yang Lein of the National Pingtung University of Science and Technology (Taiwan). Two 1 day old specific pathogen free (SPF) hybrid white leghorn chickens purchased from the Animal Health Research Institute of the Council of Agriculture (Taiwan) were used to propagate the virus by passaging 20% liver homogenates (0.1 mL per bird). The phosphate buffered saline was used to dilute the homogenates containing virus particles. These animals were inoculated orally with CIA-89 containing titers of 107.5TCID50. At 10-days post-infection, the chicken sera were collected and used to confirm the virus infection in terms of the performance of anti-CAV antibody using commercial CAV test kit (IDEXX, Netherlands). Then the chicken was sacrificed. The individual livers of the sacrificed chickens were removed, collected, immersed in formaldehyde, and then stored at room temperature until required.
The pGEX-6P-1-VP1 plasmid derived from pGEX-6P-1 plasmid (GE Healthcare, USA), which contains cDNA encoding the VP1 genes, was provided from Professor Yi-Yang Lien of National Pingtung University of Science and Technology (Taiwan) and initially used as the PCR template. To amplify the VP1 gene lacking the coding region for the first 129 amino acids from the full-length VP1 gene, two PCR primers, VP1-388FE and VP1-RHX, were designed and used as described in a previous study [8]. Using pGEX-6P-1-VP1 as the template, PCR reactions were performed at 95℃ for 5 min, 95℃ for 45 sec, 59℃ for 50 sec, and 72℃ for 1 min for 30 cycles. The last PCR cycle was carried out with a final elongation step of 10 min at 72℃. The amplified DNA fragments were digested with XhoI and EcoRI (Takara, Japan), and then cloned into the prokaryotic expression vector pET28a (Merck, Germany). The constructed recombinant plasmid, pET28a-VP1Nd129, was used to transform One Shot Top10 cells (Invitrogen, USA) and BL-21 (DE3) competent E. coli for maintaining the recombinant plasmids and for protein expression, respectively. Transformants with the correct gene size were identified by PCR and screened using restriction enzyme digestion and sequencing. The above PCR was performed in a 25 µL reaction mixture containing 0.4 mM of dNTPs, 5 pmole each of VP1-388FE and VP1-RHX, 1U Pro-taq DNA polymerase (Protech, Taiwan) and 1× Pro-taq buffer (10 mM Tri-HCl, 50 mM KCl, 0.01% gelatin, 1.5 mM MgCl2, 0.1% Triton X-100, pH 9.0). The PCR conditions was 95℃ for 5 min, followed by 35 cycles of 95℃ for 1 min, 57.7℃ for 1 min, and 72℃ for 1 min, and a final extension cycle at 72℃ for 10 min. Digestion was carried out at 37℃ for 1 h in a 50 µL reaction mixture containing 50 mM of potassium acetate, 20 mM Tris-acetate, 10 mM magnesium acetate, 1 mM DTT, 100 µg/mL BSA, 5U XhoI and 5U EcoRI. The resulting restriction digestion product was analyzed by 1% agarose electrophoresis and visualized using ethidium bromide staining and UV.
Recombinant BL-21 (DE3) E. coli containing pET28a-VP1Nd129 were used for protein induction and expression. The recombinant strains were grown overnight in Luria-Bertani (LB) medium (BD-Difco, USA) in the presence of kanamycin (50 µg/mL; Amresco, USA) at 37℃. Next, 0.5 mL of the overnight culture was used to inoculate 50 mL LB medium. The culture was grown at 37℃ for around 3 h by which time the optical density had reached 0.5. At this point, 0.1 mM isopropyl-β-D-thiogalactopyronoside (IPTG) (Amresco, USA) was added to the culture to induce protein expression, which continued for 6 h. The presence of expressed VP1Nd129 protein was confirmed by 12.5% SDS-PAGE followed by Western blotting using a monoclonal anti-His antibody (Invitrogen, USA), as described in previously work [8].
To purify the recombinant VP1Nd129 protein, a cell pellet was spun down using 5,000 × g, 15 min, at 4℃ from 50 mL of the culture supernatant and resuspended in denaturing binding buffer (20 mM NaH2PO4, 0.5 M NaCl, and 8 M urea, pH 7.8). The mixture was then sonicated on ice three times for 3 min with a 20% pulsed activity cycle (Sonics & Materials, USA), and then centrifuged for 10 min at 13,300 × g to remove cell debris. The resulting cell lysate was poured into an Enco-column (Bio-Rad, USA) with 2 mL of Ni2+-NTA agarose and the resin was allowed to settle by gravity. The packed resin in column was washed by gravity with three volumes of denaturing binding buffer and then a similar volume of wash buffer (20 mM NaH2PO4, 0.5 M NaCl, and 8 M urea, pH 6.3). Finally, the bound proteins were eluted with elution buffer (20 mM NaH2PO4, 0.5 M NaCl, and 8 M urea, pH 4). For each fraction, 2 mL of elute was collected. The fractions were monitored at OD280 using a U-2001 spectrophotometer (Hitachi, Japan) with wash buffer as a blank. Putative peaks corresponding to the recombinant CAV viral protein were identified and the eluate was collected for analysis. The total protein concentration of each fraction was determined using a Micro BCA kit (Pierce, USA) with bovine serum albumin as the reference protein. The purity of the protein samples was analyzed using aliquots of the concentrated fraction; this was done by 12.5% SDS-PAGE and Coomassie brilliant blue staining.
SPF BALB/c mice (Charles River, MA) were immunized by subcutaneous injection of 20 µg purified VP1Nd129 protein emulsified with complete Freund's adjuvant (Sigma-Aldrich, USA). After immunization, the BALB/c mice were sacrificed, the spleens were removed, and the 2 × 108 splenocytes were fused with 2 × 107 SP2/0 myeloma cells (ATCC CRL1581) by treatment with polyethylene glycol [6]. After 2 weeks of growth, the resultant hybridomas were examined the presence of antibodies against VP1Nd129 protein. Antibodies secreted from the various hybridomas were screened by ELISA. They were subcloned three times using the limiting dilution technique [20], and then ascitic fluid containing monoclonal antibodies was produced by introducing the cloned hybridomas into Pristane-primed mice (Charles River, USA). The immunoglobulin class of the hybridoma antibodies was determined by ELISA with a Mab kit (Zymed Laboratories, USA) using rabbit antisera against mouse immunoglobulin (Ig)M, IgG1, IgG2a, IgG3, and IgA, and goat anti-rabbit IgG serum conjugated with horseradish peroxidase (HRP).
To evaluate the specificity and reactivity of the mAb against CAV, an ELISA with the antibody was performed with purified VP1Nd129 protein or lysates from tissues infected with CAV as the antigen. After this, polyvinyl chloride 96-well plates were coated with recombinant VP1Nd129 or tissue lysates containing CAV. The antigen was buffered and diluted with carbonate-bicarbonate buffer (pH 9.6). Varying amounts of antigen from 1 to 500 ng were added to the 96-well strip plate and incubating overnight at 4℃. After antigen coating, the plates were washed twice with phosphate-buffered saline (PBS), containing 0.05% Tween 20 (PBS-T), and then 0.2 mL of blocking reagent (PBS containing 5% skim milk) was added to each well. After 30 min of incubation at room temperature, the plates were washed twice with PBS. One hundred µL of the hybridoma supernatants were then added to the plate wells coated with CAV antigen or recombinant antigen and incubated at 37℃ for 1 h. The bound mAbs were detected using HRP-conjugated rabbit anti-mouse IgG (Jackson Immuno Research, USA) and the color was developed with o-phenylenediamine dihydrochloride (Sigma-Aldrich, USA).
CAV-infected and uninfected liver and thymus tissues (positive and negative controls, respectively) were all from clinical cases of CAV infection confirmed by PCR using specific primers as previously described [8]. The liver and thymus samples were fixed using 30% neutral buffered formaldehyde and embedded in paraffin. The paraffin-embedded tissues were sectioned, mounted, and then stained with hematoxylin and eosin. IHC staining was carried out at room temperature. Tissue sections (5-µm thick) of the paraffin-embedded tissues were washed with phosphate saline buffer containing 0.3% hydrogen peroxide to inactivate endogenous peroxidase. After washing three times with phosphate saline buffer, antigen retrieval was carried out by incubating the tissue sections with a 0.1% of phosphate buffer diluted trypsin solution. The 250 fold diluted monoclonal antibody E3 mAb, which is specific for CAV VP1, was then applied as the primary antibody for 2 hours of the incubation. E3 mAb was recognized by HRP-conjugated goat anti-mouse IgG secondary antibody (Jackson ImmunoResearch, USA). 0.2 µg/mL of secondary antibody solution was used for 2 h of the incubation. After IHC staining, the sections were counterstained with hematoxylin and examined under light microscopy.
For IF microscopy, infected and un-infected MSB-1 cells on glass coverslips were fixed in 4% formaldehyde, washed with PBS, and permeabilized with 0.01% Triton X-100. After washing with PBS, samples were incubated with blocking solution (0.5% bovine serum albumin in PBS) for 1 h, followed by 1 h of incubation with 2 µg/mL of E3 mAb solution. 0.2 µg/mL of fluorescein isothiocyanate-conjugated goat anti-mouse IgG (Jackson Immuno-Research, USA) was used to detect E3 mAb binding. Cell nuclei were counterstained with 4',6-diamidino-2-phenylindole (Sigma-Aldrich, USA) and fluorescence images were captured using a laser scanning confocal microscope (Leica Microsystems, Germany).
To create an immunoaffinity column, cytoplasmic extracts from chicken liver tissues infected with CAV were prepared by centrifugation at 13,300 × g for 30 min at 44℃. Next, 100 µL of cell extract were mixed with 50 µL of ascitic fluid and incubated overnight at 4℃. Then, 50 µL of protein A agarose beads (Sigma-Aldrich, USA) were added and incubated overnight at 44℃ with gentle rotation. The agarose beads were washed three times with buffer containing 50 mM Tris-HCl (pH 7.5), 500 mM NaCl, 0.2% NP-40, and 0.05% sodium deoxycholate. Finally, viral protein and/or CAV virus particles were eluted from the protein A agarose beads by boiling with SDS-PAGE sample buffer (60 mM Tris-Cl pH 6.8, 2% SDS, 10% glycerol, 5% β-mercaptoethanol, 0.01% bromophenol blue) for 5 min. The extracted proteins were then subjected to SDS-PAGE followed by Western blotting using E3 mAb as primary antibody and HRP-conjugated goat anti-mouse IgG secondary antibody, respectively. In addition, CAV genomic DNA from the CAV virus particles was detected by PCR using CAV genome-specific primers. The sequences of the CAV VP1 gene PCR primers were VP1F: 5'-ATGGCAAGACGAGCTCGCAGACCGAGAGG-3' and VP1R: 5'-CTAACCATGGTGATGGTGATGGTGGGGCTGCGTCCCCCAGTA-3'. The sequences of the CAV VP3 primers were VP3F: 5'-CCGCTCGAGCAGTCTTATACACCTTCTTG-3' and VP3R: 5'-GCGAATTCATGAACGCTCTCCAAGAAGATAC-3'. The PCR conditions was 95℃ for 5 min, followed by 35 cycles of 95℃ for 1 min, 57.7℃ for 1 min, and 72℃ for 1 min, and a final extension cycle at 72℃ for 10 min.
To express the CAV VP1 as an antigen for immunization, the VP1Nd129 construct was created by PCR using VP1 cDNA as the template DNA. The VP1 cDNA partially overlapped partially the VP2 gene of the CAV genome, which had been previously cloned into the pGEX-6P-1 plasmid [4]. Using the primers VP1-388FE and VP1-RHX, the VP1Nd129 construct was amplified by PCR and cloned into the pET28 a vector at the EcoRI and XhoI sites, thereby creating a protein with an in-frame His-tag. This plasmid, pET28a-VP1Nd129, was used to transform E. coli BL-21 (DE3) cells. The E. coli were examined for protein expression after 4 h of induction with IPTG. VP1Nd129 protein was successfully expressed in E. coli, as noted by the correct size band on a gel stained with Coomassie blue gel and recognition by anti-His tag antibodies (Fig. 1). The estimated molecular weight of pET28a-VP1Nd129 was 40 kDa. VP1Nd129 in E. coli was expressed at a high level which reached 26.2 mg/L when 0.1 mM IPTG was added to the culture broth. After affinity chromatography purification, the purified VP1Nd129 protein was confirmed by Western blotting using anti-His and anti-CAV antibodies. The purity of VP1Nd129 approached homogenicity in elution fractions E1 to E7 at pH 4.0. Purification of His-tag-fused VP1Nd129 was therefore feasible using a Ni-NTA column. Additionally, the identity of the Ni-NTA-purified VP1Nd129 protein was confirmed by mass spectrometry (data not shown).
To establish monoclonal antibodies, spleen cell were removed from five mice immunized with Ni-NTA-purified recombinant VP1Nd129 protein and fused with SP2/0 myeloma cells. After 14 days, hybridoma cell lines secreting mAbs specific for VP1Nd129 were screened by ELISA using the Ni-NTA-purified VP1Nd129 protein as the coating antigen. Four mAbs were found active against VP1Nd129 (B1-1, B1-5, D4 and E3) were identified out of 64 clones; these were then screened by subcloning at least three times using the limiting dilution method. The mAb from the E3 hybridoma cells showed the greatest reactivity compared to the other mAbs from the B1-1, B1-5, and D4 hybridomas (data not shown). Even after the supernatant was diluted 50-fold, E3 still has recognition activity to react the antigen. In addition, the specificity and reactivity of the E3 mAb were also confirmed by Western blotting using purified recombinant VP1Nd129 and E. coli cell lysates. As illustrated in Fig. 2A, the E3 mAb showed good specificity and reactivity against the antigen and purified recombinant VP1Nd129 while showing no reaction against cell lysates from E. coli harboring the blank pET28a vector.
When the specificity of the E3 mAb against different amounts of antigens was evaluated, it was found to be constant and the reactivity of the E3 mAb increased with increasing amounts of antigen. There were very few background bands that reacted with E3 mAb in either the samples of purified VP1Nd129 or the E. coli cell lysate. Moreover, 1 ng of purified VP1Nd129 was sufficient to react with E3 mAb (Fig. 2B). Thus, E3 mAb has good specificity and reactivity against CAV antigen. Additionally, IgG subtype analysis showed that E3 mAb was IgG2b with a κ light chain. At this point, the E3 hydridoma was selected to produce mAbs in mice. Mouse ascitic fluid was then used for further diagnostic applications and characterization.
To evaluate the possible clinical application of E3 mAb for CAV diagnosis, the mAb was used for CAV detection by IHC and immunochromatographic assay. First, four CAV-infected chicken livers were analyzed using IHC; all of the paraffin-embedded tissues were positive. The positive control slides demonstrated strong immunoreactivity against CAV antigen (Fig. 3B). In addition, four CAV mock-infected chicken livers were analyzed as the negative control; these were all negative for immunoreactivity against CAV antigen, (Fig. 3A). Moreover, similar immunoreactivity results were found in the thymus tissues. The CAV-infected thymus shown in Fig. 4B was recognized by the E3 mAb using IHC in contrast to the negative sample (Fig. 4A).
In CAV-infected MSB-1 cells and CAV-infected MSB-1 showing a CPE, the presence of VP1 antigen in the cells was recognized by the E3 mAb using an IF assay (Fig. 5). These results indicated that E3 mAb was able to discriminate CAV-infected tissues or cells from uninfected tissue or cells in experimental samples using IHC staining and IF, respectively. Furthermore, E3 mAb was used to react with VP1 present in the lysate of CAV-infected liver tissue. As illustrated in Fig. 6A, VP1 with a molecular weight of 50 kDa was eluted from the protein A agarose beads. In contrast, no VP1 was detected from the eluted sample of CAV mock-infected liver lysate. Detection of the protein was performed by Western blotting using E3 mAb as the primary antibody. In addition, Fig. 6B shows that CAV-specific VP1 and VP3 genes with lengths of 1.35 kb and 348 bp, respectively, were amplified by PCR using the eluted fraction from the immunochromatographic column as a template. This suggests that the eluted fraction from the immunochromatographic column included the CAV VP1 capsid.
In this study, we successfully generated a specific monoclonal antibody, E3 mAb, which is active against VP1, the sole structural protein of CAV. To generate monoclonal or polyclonal antibodies against VP1, purified CAV particles are usually used as the main antigen for producing the antibodies. However, virion purification is very tedious and time-consuming. Therefore, DNA recombinant technology was chosen as a better method for producing VP1.
Previously, several expression systems have been used to express CAV VP1, including E. coli, baculovirus-insect cells, and plant cells [5,6,15]. However, expressing full-length VP1 protein has been difficult because of cytotoxicity [15]. Moreover, production of the recombinant full-length VP1 protein has generally been unsuccessful because of a failure to express a span of amino acids at the N-terminus that is highly rich in arginine residues [8,15]. This might because these amino acids are encoded by codons that are rarely used by E. coli. Most viral capsid proteins of circoviruses such as porcine circovirus and pigeon circovirus including the rare codons of E. coli at N-terminus have been investigated in previous studies. Truncation of the N-terminus of capsid proteins has therefore become an alternative way to produce these recombinant viral proteins [2,9]. Thus, as can be resolved using above truncated recombinant protein, monoclonal antibodies or detection kit could be generated [2,4,5,9,13]. Lee et al. [8] reported that the expression of VP1 in a host system can be vastly improved when the arginine residues at the VP1 N-terminus are removed. Using this N-terminus-truncated clone of VP1 expressed in E. coli, we successfully produced and purified VP1Nd129 protein. After using this antigen to create E3 mAb against VP1, this antibody was evaluated in terms of its use in IHC, IF, and immunoaffinity chromatographic column purification. Several diagnostic methods have been developed and refined for detecting CAV infection including electron microscopy, nucleic acid-based analysis, and antigen-antibody assays [11,12,17,18]. IF assays have been most commonly used for diagnosing CAV infections. Combining IF and confocal laser scanning microscopy would establish a highly convenient method for directly investigating CAV biology at a subcellular level.
It is well known that VP1 is the sole structural protein of CAV; thus this protein is a highly useful marker for detecting CAV. In the life cycle of CAV, VP1 encapsidates the CAV genome during virus particle assembly [16]. The fact that VP1 capsid was eluted from the protein A agarose beads in this study implies that the CAV genome co-eluted with the VP1 protein. When CAV virions purified by immunoaffinity column were analyzed by PCR, CAV-specific gene amplification was observed, which confirmed the presence of assembled virus particles.
Epitope mapping of VP1 in terms of mAb recognition was not performed in this study. However, mAb E3 might recognize a specific epitope that is located on the surface of VP1 particles. Thus, it is possible that E3 mAb might recognize a neutralizing epitope and the antibody could as a virus neutralizing antibody. This could be determined in the future by means of a virus neutralization assay and would help with the development of a peptide vaccine for controlling CAV infections.
In conclusion, we generated monoclonal antibodies against recombinant VP1Nd129 protein using a mouse hybridoma system. The most immunodominant epitope, VP1 of the CAV capsid, was detected immunologically using one of these monoclonal antibodies, E3 mAb. This monoclonal antibody enabled the visualization of the viral antigen in formalin-fixed tissue samples by IHC. In the absence of virus recovery, diagnosing CAV infection could be achieved by an immunological approach using E3 mAb for antigen detection. Therefore, the immunological probe generated in this study represents a powerful tool that will promote the development of CAV diagnostic systems for epidemiological investigations. In addition, these systems will assist with measuring the immunization efficacy of vaccines, enhance the study of CAV biology, and enable general surveillance of CAV infections.
Figures and Tables
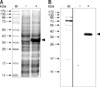 | Fig. 1Analysis of the VP1Nd129 protein expressed in recombinant Escherichia (E.) coli by SDS-PAGE (A) and Western blotting (B). The symbols '-' and '+' represent pre- and post-induction with 1 mM of isopropyl-β-D-thiogalactopyronoside in E. coli, respectively. Anti-His tag monoclonal antibody was used for recognizing the VP1Nd129 protein. The arrowheads are the symbol for expressed VP1Nd129 protein. |
 | Fig. 2Analysis of the specificity and reactivity of E3 monoclonal antibody (mAb) by Western blotting (A) and ELISA (B). Different amounts of purified VP1Nd129 or E. coli cell lysate were reacted with E3 mAb in both (A) and (B). |
 | Fig. 3Immunohistochemical labeling of chicken anemia virus (CAV)-infected chicken liver tissues. E3 mAb was used as the primary antibody for recognizing CAV antigen in liver tissue. (A) Negative control. (B) CAV-infected chicken liver tissue. Scale bars = 100 nm. |
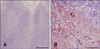 | Fig. 4Immunohistochemical labeling of CAV-infected chicken thymus tissues. E3 mAb was used as the primary antibody for recognizing CAV antigen in the thymus tissue. (A) Negative control. (B) CAV-infected chicken thymus tissue. Scale bars = 100 nm. |
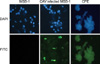 | Fig. 5Immunofluorescence assay of CAV-infected chicken MSB-1 cells. E3 mAb was used as the primary antibody for recognizing CAV VP1 antigen in the MSB-1 cells. The fluorescent agent 4',6-diamidino-2-phenylindole (DAPI) and fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (IgG) were used to counterstain the cell nuclei of cultures and react with E3 mAb, respectively. CPE: CAV-infected MSB-1 cells showing a cytopathic effect. |
 | Fig. 6Western blotting of immunocolumn-purified CAV VP1 by E3 mAb (A). CAV-infected liver lysate reacted with E3 mAb and immunoaffinity resin was then used to precipitate the mAb-bound or unbounded antigen. Lane M: pre-stained protein marker (the molecular weights of respective bands are 116, 66, 45, 35, 25, and 18 kDa), Lane 1: uninfected liver lysate, Lane 2: CAV-infected liver lysate, Lane 3: VP1 protein co-precipitated with E3 mAb from tissue lysate of Lane 2, Lane 4: E3 mAb only, Lane 5: protein precipitated by E3 mab from tissue lysate of Lane 1. "H" and "L" indicate the IgG heavy and light chains, respectively. Identification of the CAV genome by PCR (B). CAV VP1 capsid was co-precipitated with E3 mAb and then used to as a DNA template for PCR amplification using primers specific for the VP1 and VP2 genes. Lane M: 1-kb DNA ladder marker, Lane 1: positive PCR control, Lane 2: uninfected liver lysate, Lane 3: CAV-infected liver lysate, Lane 4: sample co-precipitated with E3 mAb from CAV-infected liver lysate. |
References
1. Brentano L, Lazzarin S, Bassi SS, Klein TAP, Schat KA. Detection of chicken anemia virus in the gonads and in the progeny of broiler breeder hens with high neutralizing antibody titers. Vet Microbiol. 2005. 105:65–72.

2. Daum I, Finsterbusch T, Härtle S, Göbel TW, Mankertz A, Korbel R, Grund C. Cloning and expression of a truncated pigeon circovirus capsid protein suitable for antibody detection in infected pigeons. Avian Pathol. 2009. 38:135–141.

3. Ducatez MF, Owoade AA, Abiola JO, Muller CP. Molecular epidemiology of chicken anemia virus in Nigeria. Arch Virol. 2006. 151:97–111.

4. Huang CH, Lai GH, Lee MS, Lin WH, Lien YY, Hsueh SC, Kao JY, Chang WT, Lu TC, Lin WN, Chen HJ, Lee MS. Development and evaluation of a loop-mediated isothermal amplification assay for rapid detection of chicken anaemia virus. J Appl Microbiol. 2010. 108:917–924.

5. Iwata N, Fujino M, Tuchiya K, Iwata A, Otaki Y, Ueda S. Development of an enzyme-linked immunosorbent assay using recombinant chicken anemia virus proteins expressed in a baculovirus vector system. J Vet Med Sci. 1998. 60:175–180.

6. Köhler G, Milstein C. Derivation of specific antibodyproducing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976. 6:511–519.

7. Lacorte C, Lohuis H, Goldbach R, Prins M. Assessing the expression of chicken anemia virus proteins in plants. Virus Res. 2007. 129:80–86.

8. Lee MS, Lien YY, Feng SH, Huang RL, Tsai MC, Chang WT, Chen HJ. Production of chicken anemia virus (CAV) VP1 and VP2 protein expressed by recombinant Escherichia coli. Process Biochem. 2009. 44:390–395.

9. Lou Z, Li X, Li Z, Yin X, Li B, Lan X, Yang B, Zhang Y, Liu J. Expression and antigenicity characterization for truncated capsid protein of porcine circovirus type 2. Can J Vet Res. 2011. 75:61–64.
10. Lucio B, Schat KA, Shivaprasad HL. Identification of the chicken anemia agent, reproduction of the disease, and serological survey in the United States. Avian Dis. 1990. 34:146–153.

11. McNulty MS, Connor TJ, McNeilly F, Spackman D. Chicken anemia agent in the United States: isolation of the virus and detection of antibody in broiler breeder flocks. Avian Dis. 1989. 33:691–694.

12. McNulty MS, Curran WL, Todd D, Mackie DP. Chicken anemia agent: an electron microscopic study. Avian Dis. 1990. 34:736–743.

13. Meehan BM, Todd D, Creelan JL, Earle JA, Hoey EM, McNulty MS. Characterization of viral DNAs from cells infected with chicken anaemia agent: sequence analysis of the cloned replicative form and transfection capabilities of cloned genome fragments. Arch Virol. 1992. 124:301–319.

14. Noteborn MHM, de Boer GF, van Roozelaar DJ, Karreman C, Kranenburg O, Vos JG, Jeurissen SHM, Hoeben RC, Zantema A, Koch G, van Ormondt H, van der Eb AJ. Characterization of cloned chicken anemia virus DNA that contains all elements for the infectious replication cycle. J Virol. 1991. 65:3131–3139.

15. Pallister J, Fahey KJ, Sheppard M. Cloning and sequencing of the chicken anaemia virus (CAV) ORF-3 gene, and the development of an ELISA for the detection of serum antibody to CAV. Vet Microbiol. 1994. 39:167–178.

17. Todd D, Creelan JL, McNulty MS. Dot blot hybridization assay for chicken anemia agent using a cloned DNA probe. J Clin Microbiol. 1991. 29:933–939.

18. Todd D, Mawhinney KA, McNulty MS. Detection and differentiation of chicken anemia virus isolates by using the polymerase chain reaction. J Clin Microbiol. 1992. 30:1661–1666.

19. Wang X, Gao H, Gao Y, Fu C, Wang Z, Lu G, Cheng Y, Wang X. Mapping of epitopes of VP2 protein of chicken anemia virus using monoclonal antibodies. J Virol Methods. 2007. 143:194–199.

20. Wewetzer K, Seilheimer B. Establishment of a single-step hybridoma cloning protocol using an automated cell transfer system: comparison with limiting dilution. J Immunol Methods. 1995. 179:71–76.

21. Yuasa N, Taniguchi T, Imada T, Hihara H. Distribution of chicken anemia agent (CAA) and detection of neutralizing antibody in chicks experimentally inoculated with CAA. Natl Inst Anim Health Q (Tokyo). 1983. 23:78–81.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download