Introduction
Herpes simplex virus type 1 (HSV-1)-based vectors have several features that make them a very useful tool for efficient gene delivery. These vectors have the capacity to package and deliver up to 150 kbp of foreign DNA to the nucleus of most proliferating and quiescent mammalian cells [6]. The viral genome contains more than 80 genes of which approximately half are nonessential for viral replication and can therefore be deleted without disturbing virus production in cultured cells [7]. Neurotropic HSV-1 can naturally infect a large number of cell types. Two types of vectors can be derived from HSV-1: amplicon vectors (vAs) and recombinant vectors [6]. The present study focused only on amplicon vectors. HSV-1 amplicons are packaged bacterial plasmids containing two non-coding genetic elements from HSV-1, an origin of DNA replication, and a packaging/cleavage signal (a) which allow amplicon replication and packaging into HSV-1 particles [6]. In the presence of a suitable helper virus, or a helper virus genome, the amplicon plasmid (pA) is replicated and packaged as a DNA concatemer into HSV-1 virions [9,22]. Therefore, a variable number of copies of the transgene sequence will be packaged depending on the size of the amplicon plasmid. This could result in the delivery of multiple copies of the foreign gene to each individual cell that is infected by an amplicon particle, thus resulting in high expression levels [2,8].
Bovine herpesvirus 1 (BHV-1) is a pathogen of major economic importance in the cattle industry worldwide. BHV-1 is the causative agent of respiratory infection (infectious bovine rhinotracheitis, IBR), genital infection (infectious pustular vulvovaginitis), conjunctivitis, and systemic infection leading to abortion and fetal death [29]. IBR also facilitates superinfection of cattle by bacterial agents, resulting in cases of bronchitis and/or pneumonia that are frequently fatal if not treated [21]. Vaccination is an effective method for controlling IBR but current vaccines have not been completely successful. Modified live vaccines may cause abortions, immunosuppresion, and the establishment of latent infection [26,27] while killed vaccines do not provide complete protection even following the administration of two doses [19]. Efforts are being made to use viral vectors for efficient antigen delivery to cattle [11]. These strategies usually focus on three major envelope glycoproteins: glycoprotein (g)B, gC, and gD. These glycoproteins play key roles in the early steps of infection and are major targets for both cellular and humoral immunity [20]. One of the three glycoproteins, gD, has been proposed as the principal vaccine candidate since it induces a more consistent and stronger cellular immune response than the others. Additionally, antibodies against gD have the highest neutralizing titers [5,9]. This protein is a typical transmembrane glycoprotein of 417 amino acids that includes a signal sequence of 18 amino acids [22] which is cleaved during processing to yield a mature protein of 399 amino acids with a molecular mass of 71 kDa [25].
The purpose of this study was to clone and express the full-length of gD and truncated form of gD (gDtr) which lacks the trans-membrane region of BHV-1 using an HSV-1 amplicon vector system, and to assess the immune response generated by inoculating a murine model with these vectors.
Materials and Methods
Cells
Vero-7b (Vero-derived cell line expressing ICP4/ICP27) [12], Gli36 (glioblastoma cell line) [10], and Madin-Darby bovine kidney (MDBK) cells were cultured in Dulbecco's minimum essential medium (DMEM; Invitrogen, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen, USA), and 1% antibiotic-antimycotic solution (Invitrogen, USA) composed by 100 units/mL of penicillin G, 100 µg/mL of streptomycin, and 0.25 µg/mL of amphotericin B. Vero-7b cells were selected with 1 mg/mL of Geneticin (Invitrogen, USA) every three passages. Escherichia coli DH5α cells (New England Biolabs, USA) were used for cloning experiments and plasmid propagation. Bacterial strains were routinely grown at 37℃ in Luria-Bertani broth (Difco, USA) or on agar containing medium and supplemented with 100 µg/mL ampicillin (MP Biomedicals, France).
Viruses
A defective cre-loxP based helper virus (HSV-1 LaLΔJ) was previously constructed in Alberto Epstein's Laboratory [29]. This is a defective HSV-1 virus used as helper to produce amplicon vectors that was propagated and titrated in Vero-7b cells. Virus stock was produced in roller bottles containing 1 × 108 Vero-7b cells infected at multiplicity of infection (MOI) of 0.1 plaque forming unit (PFU)/cell in Medium 199 (Invitrogen, USA) supplemented with 1% FBS (M199 1% FBS). When a complete cytophatic effect (CPE; round cells forming grape-like clusters) was observed (48~72 h post-infection), the virus was harvested and concentrated using the following technique. A first round of centrifugation at 1,000 × g for 10 min at 4℃ was done to remove the cells. The pellet was diluted in 400 µL of M199 1% FBS and frozen/thawed three times to break down the infected cells and facilitate the viral particles release .The pellet solution was clarified at 1,000 × g for 10 min at 4℃ and we kept the supernatant (named solution A). The supernatant from the first round containing viral particles was centrifuged at 18,000 × g for 1 h at 4℃ and the pellet obtained was resuspended with solution A. This final solution was aliquoted and stored at -80℃ until use The titer of HSV-1 LaLΔJ stock was determined by a plaque assay [29]. Vero-7b cells were infected with serial dilutions of viral stock and incubated with M199 1% FBS and 1% carboxymethylcellulose (Sigma, USA). The HSV-1 LaLΔJ titer was calculated by counting plaques formed in the monolayer at 3 days post infection.
The BHV-1 strain (provided by Santa Elena Laboratory, Uruguay) used for an enzyme-linked immunosorbent assay (ELISA) and neutralization assays was propagated in MDBK cells. Confluent MDBK monolayers were inoculated with BHV-1 at a MOI of 0.05 PFU/cell and the cells were allowed to adsorb the virus for 1 h at 37℃ before the addition of DMEM 1% FBS. Once a complete CPE was observed (48~72 h post-infection), we proceeded to harvest and concentrate the BHV-1 virus stock as described above. In a second stage, BHV-1 production was filtered through 0.45 µm sterile filter and the virions were concentrated by centrifugation through a 25% sucrose cushion. Viral pellet was resuspended in PBS and titrated in MDBK cells by plaque assay [13].
Plasmids
Construction of pAgD BHV-1
Amplicon plasmid pAEUA2 [1] containing one HSV-1 replication origin and one HSV-1 package signal (a) was used to derive the amplicon plasmid pAgD BHV-1 expressing full-length gD (Fig. 1). In addition, pAEUA2 expressed enhanced green fluorescent protein (EGFP) under the control of the HSV-1 immediate-early promoter IE4/5, which was used to titrate the vectors and as reporter gene to identify infected cells. The construct also contained a multiple cloning site (MCS) surrounded by the human immediate-early cytomegalovirus (HCMV) promoter and SV40 polyadenylation site where the open reading frame (ORF) of interest was cloned. First, pAEUA2 was linearized and the blunt ends were ligated into the XbaI site at the MCS. The gD BHV-1 gene, obtained by digestion with EcoRI and HindIII from pCS133 (kindly provided by Dr. Cornell Fraefel, University of Zurich, Switzerland), was cloned into the XbaI site at the MCS of pAEUA2, thus producing the pAgD BHV-1 amplicon plasmid.
Construction of pAgDtr BHV-1
gDtr was amplified from pCS133 using the forward primer 5'-CTAGGCTAGCAGCTTATGCAAGGGCCGACAT-3' (modified from the primer dgDFRw [23] containing the gene start codon) and the reverse primer 5'-CTAGTCTAGATCAGTCGGGGGCCGCGGGCGTAG-3' (modified from the primer 1161RgD [3] containing one ectopic stop codon to delete the transmembrane region). This fragment was subcloned into the geneJET vector (Fermentas, USA) and finally cloned into pAEUA2 digested with NheI at the MCS. Plasmid constructions were confirmed by DNA restriction assays and sequence analysis.
Amplicon vectors
Amplicon vector stocks were produced as previously described [29]. Briefly, 60~70% confluent Vero-7b cells seeded in F75 flasks (Nunc, Denmark) were transfected with the amplicon plasmids using Lipofectamine and Plus Reagent (Invitrogen, USA) according to the manufacturer's protocol. At 24 h, cells were superinfected with HSV-1 LaLΔJ at a MOI of 0.3 PFU/cell and incubated in M199 1% FBS until a 100% CPE was observed. This stock (named P0) was harvested as described for HSV-1 LaLΔJ. To amplify the amplicon vector stocks, P0 was used to infect Vero-7b cells seeded in F175 flasks at a MOI 0.3 PFU/cell, thus generating P1 progeny. P1 was amplified further by infecting Vero-7b cells grown in roller bottles to generate a high-titer P2 amplicon vector stock. After each production, amplicon virus titers expressed as transducing units per mL (TU/mL) were determined by counting EGFP-expressing Gli36 cells while helper virus titers were determined by plaque assay in Vero-7b cells and expressed as PFU/mL.
Detection of BHV-1 gD expressed in Gli36 cells
An immunofluoresence assay was done to visualize the expression of gD from both plasmids. Gli-36 cells seeded in 8-well Lab-Tek chamber slides (Nunc, Denmark) were transfected with Lipofectamine and Plus reagent (Invitrogen, USA) either with pAEUA2 (control), pAgD BHV-1, or pAgDtr BHV-1 according to the manufacturer's recommendations. After 24 h, the cells were washed twice with PBS and fixed with 3% paraformaldehyde in PBS for 20 min at room temperature. The cells were then permeabilized with 1% Triton X-100 in PBS for 5 min. The slides were incubated for 2 h at room temperature with blocking buffer [1% bovine serum albumin (BSA) and 0.5% Tween 20 in PBS] containing 3% FBS, and then for 1 h with the monoclonal antibody 1106 diluted 1 : 10 [17] (kindly provided by Dr. Cornel Fraefel, University of Zurich, Switzerland) in blocking buffer plus 1% FBS. Goat anti-mouse IgG H+L Alexa Fluor 555 (Invitrogen, USA) was used as the secondary antibody. The slides were incubated with the secondary antibody diluted 1 : 2,000 in the same solution as the primary antibody for 45 min. Subsequently, the slides were washed three times with PBS containing 10 mM glycine and mounted in glycerol containing 1,4-diazabicyclo [2.2.2] octane (Sigma, USA) and 4', 6-diamidino-2-phenylindole (Sigma, USA) to stain nuclei.
Analysis of immune response in mice infected with vAgD-BHV-1 and vAgDtr-BHV-1 amplicon vectors
Six 8-week-old BALB/c female mice were bred and maintained under standard conditions in the specific pathogen-free animal facilities of the Institut Pasteur of Montevideo, Uruguay. Four groups of three mice were injected intramuscularly with 5 × 105 TU/mL of amplicon vector. The different groups received (a) PBS; (b) the control amplicon vector pAEUA2 which expressed no gD; (c) amplicon vector pAgD BHV-1 expressing full-length gD; and (d) amplicon vector pAgDtr BHV-1 expressing gDtr. All groups were boosted at 21 days post-vaccination.
Mice blood was collected on days 0, 21, 36 and 43 post-vaccination by a submandibular route and the sera were stored at -20℃ until use. BHV-1-specific antibody titers were determined by an ELISA using BHV-1 virus particles as the antigen. Ninety-six-well immunoplates (Dynex Technologies, USA) were coated with 50 µL (corresponding to 2 × 104 PFU/well) of the virus diluted in PBS or a control preparation in PBS for 16 h at 4℃. A saturation step was performed by adding 200 µL/well of PBS-1% BSA and incubating the plates for 90 min at room temperature. The plates were washed three times with PBS-0.05% Tween 20. Mice sera (50 µL/well) were serially diluted two-fold in PBS-0.05% Tween 20-0.1% BSA. Serum from mice injected with PBS and pAEUA2 vector were used as controls. The positive control was ascitic fluid from monoclonal antibody 4~214 (diluted 1 : 3,000) developed against IBR gD (kindly provided by Dr. Cristina Seki from CEVAN-CONICET, Argentina).
After 1 h of incubation at 37℃, the plate was washed three times with 0.05% Tween 20 in PBS and the plate was incubated with biotinylated goat anti-mouse IgG (GE Healthcare, UK) diluted 1 : 5,000 for 1 h at 37℃. The plate was then washed three times with 0.01% Tween 20 in PBS followed by a 30-min incubation in the dark with streptavidin-HRP (GE Healthcare, UK) diluted 1 : 500 in 0.1% BSA in PBS. After five washes with PBS, the reaction was developed using o-phenylenediamine-H2O2 (Sigma, USA) in citrate-phosphate buffer (pH 5) during 20 min at room temperature. The reaction was stopped by addition of 50 µL 3N H2SO4 and the absorbance was measured at 492 nm in a Varioskan Flash microplate reader (Thermo Scientific, USA). Antibody titer was expressed as the reciprocal of the highest dilution resulting in a reading of two standard deviations above the control value.
BHV-1 glycoprotein D-specific antibody responses were evaluated by a Western immunoblot using semi-purified BHV-1 as antigen. Briefly, the antigen was incubated in sample buffer (63 mM Tris-HCl, 2% SDS, 10% glycerol, 0.01% bromophenol blue, and 0.1M β-mercaptoethanol) and boiled for 5 min. The proteins were separated on a 12% acrylamide SDS-polyacrylamide electrophoresis gel. The proteins were then transferred to a mixed ester nitrocellulose membrane (Hybond-C; GE Healthcare, UK) and subjected to Western blotting in order to detect BHV-1 gD. A pool of mouse sera diluted 1 : 20 was used as the primary antibody which was detected with a peroxidase-conjugated secondary anti-mouse antibody (Santa Cruz Biotechnology, USA). Both primary and secondary antibodies were diluted in BSA 3% Tween 20 0.3% in PBS and incubated for 1 h at room temperature. Finally, the bands were visualized using SuperSignal West Pico chemiluminescent (Pierce, USA) substrate after exposure to radiographic film (GE Healthcare, UK).
The neutralizing activity of mouse serum antibodies was analyzed by a plaque reduction assay in MDBK cells. The cells were seeded in 48-well plates at a density of 1 × 105 cells/well in DMEM supplemented with 10% FBS. The following day, 25 µL of mouse sera were incubated with 25 µL (20 PFU) of BHV-1 for 1 h at 37℃. Positive (guinea pig hyperimmune serum against BHV-1) and negative (guinea pig non immune serum) controls were included. The MDBK cells were washed with PBS and incubated with 50 µL of mouse sera incubated with BHV-1 for 1 h at 37℃. After this, fresh M199 1% FBS and 1% carboximethil-cellulose were added. Two days later, the plates were fixed with 4% formaldehyde in 0.15 M NaCl and stained with crystal violet. Plaques in the plates were counted. The neutralization antibody titers were expressed as the highest dilution of serum that reduced the number of plaques by 50% relative to the virus control.
For statistical analysis, Student's t-test was performed using Stata/SE 10.0 (Stata Corporation, USA). A p value <0.05 was considered significant.
Results
Generation of amplicon plasmids expressing gD and gDtr BHV-1
Two different amplicon plasmids expressing two forms of BHV-1 gD were generated using the plasmid pAEUA2 [1] as described in Materials and Methods. Briefly, the whole or the truncated ORF of each form of gD were cloned downstream of the constitutive promoter HCMV of pAEUA2 to generate the plasmids pAgD BHV-1 and pAgDtr BHV-1, respectively (Fig. 1). pAgD BHV-1 expressed the entire coding region of BHV-1 gD, allowing the glycoprotein to be expressed as a membrane anchored protein. The gD form expressed by pAgDtr BHV-1 lacked the trans-membrane region and should have therefore been secreted from the vector-infected cells.
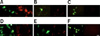
To assess whether these plasmids behaved as expected, Gli36 cells were transfected with each plasmid and gD expression was evaluated by indirect immunofluorescence (Fig. 2). pAgD BHV-1 led to the efficient expression of gD in Gli36 cells at 24 h post-transfection. The expressed protein was localized in the cytoplasm around the nucleous and at the plasma membrane as expected (Fig. 2A). In contrast, gDtr, the truncated form of gD expressed by pAgDtr BHV-1, was present in the cytoplasm and formed aggregates that are characteristic of secreted proteins (Fig. 2B). Since gDtr was not detected in the plasma membrane, we concluded that it was being secreted out of the cell. We were not able to perform a Western blot analysis as our monoclonal antibodies did not work for this assay. No gD was observed in cells transfected with the control pAEUA2 plasmid (Fig. 2C). In all cases, cells expressing gD also expressed the reporter protein EGFP.
Generation of amplicon vectors expressing gD and gDtr BHV-1
The amplicon plasmids described above were used to generate two amplicon vectors, vAgD BHV-1 and vAgDtr BHV-1. The backbone plasmid pAEUA2 was used to generate a control vector that expressed EGFP but not gD. The reporter gene EGFP, expressed by the three vectors, permitted us to titrate the amplicon vector stocks by counting green fluorescent cells with a fluorescence microscope. EGFP expression also allowed us to identify the infected cells. All the vector stocks produced had titers between 107~108 transducing units/mL.
Gli36 cells were infected at a MOI of 1 PFU/cell with the vectors. As shown in Figs. 2D and E, vectors encoding gD expressed the expected forms of the glycoprotein in a pattern identical to that observed in cells transfected with the corresponding amplicon plasmids. No gD was observed in cells infected with the control vAEUA2 vector (Fig. 2F). The same results were obtained with MDBK cells (data not shown). Taken together, these results show that the amplicon vectors can be used to efficiently express the desired antigenic forms of BHV-1 gD.
Immune response in mice inoculated with amplicon vectors expressing gD
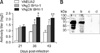
The ability of exogenously expressed gD and gDtr to elicit antibody responses following intramuscular inoculation of mice was evaluated by an ELISA. As shown in Fig. 3A, both gD and gDtr amplicon vectors induced gD-specific IgG responses. At 21 days post-infection (dpi), the levels of anti-BHV-1 antibodies were very similar between mice inoculated with gD or gDtr. Following administration of a boost at day 21, the antibody response increased significantly compared to that of the control and was much higher for mice that received gDtr than ones given gD (36 and 43 dpi, and 15 and 21 days after the second dose administration, respectively).
Additionally, specific anti-BHV-1 gD antibodies were detected in the sera from mice immunized with vAgD BHV-1 and vAgDtr BHV-1. As show in Fig. 3B, the pool sera from day 36 p.i. recognized a band with a size expected for glycoprotein D (71 kDa). The herpes amplicon vector containing the gDtr (vAgDtr BHV-1) produced the highest level of antibody response in correlation with the antibody titer determined by an ELISA. The neutralizing activity measured by reduction plaque assay showed that mice sera were unable to protect against BHV-1 infection.
Discussion
In the present study we constructed HSV-1 amplicon vectors expressing either BHV-1 gD or gDtr proteins and evaluated the immunogenicity induced by these constructs in mice. We cloned the entire gD gene of BHV-1 into the pAEUA2 amplicon genome. In parallel, we cloned a gDtr lacking the transmembrane anchor which would allow secretion of gD into the extracellular environment.
Both forms of the gD proteins were detected in cells transfected with the amplicon plasmids and cells infected with the amplicons vectors. Furthermore, the distribution patterns for each form of gD, resulting from transfection of plasmids or infection with the vectors, greatly differed. The ELISA results showed that vectors vAgD BHV-1 and vAgDtr BHV-1 induced the production of statistically significant levels of anti-BHV-1 antibodies in BALB/c mice. This response was against BHV-1 gD as confirmed by Western blotting. This result was expected since gD dominant epitopes are maintained in the gDtr protein [25]. In particular, antibody levels generated by vAgDtr BHV-1 were higher than those generated by vAgD BHV-1 after the second dose. This response could indicate that the truncated form of gD was properly secreted into the extracellular medium and became more easily detectable by the immune system, resulting in an enhanced humoral response. Moreover, we cannot discard the possibility that the different level of antibody response in the mice was the result of higher protein expression from vAgDtr BHV-1 compared to vAgD BHV-1. It is also important to note that we observed unspecific low reactivity with other proteins in all sera. This can be explained by the partial purification of BHV-1 antigen carrying cell proteins that reacted with the serum from mice.
Consistent with our results, previous studies have shown that plasmid DNA encoding gDtr elicits a greater immune response than the same plasmid vector encoding gD in mice and cattle immunized via an intradermal route [1,16-18,27]. However, the response against gD and gDtr was similar in both mice and cattle when the animals were immunized by an intramuscular route [14-16,24]. Using recombinant viral vectors expressing BHV-1 gD, several reports have demonstrated an effective immune response against BHV-1 [5,6,13]. However, there are no studies using viral amplicon vectors, which have the advantage of expressing no viral proteins other than the desired antigens. Furthermore, recombinant bovine adenovirus type 3 used to express gD and gDtr in cotton rats immunized intranasally was found to induce a lower humoral immune response to gD than to gDtr [28]. Yet, intradermal immunization of cotton rats with recombinant human adenovirus type 5 (HAV-5) elicits a lower humoral immune response to gDtr than gD [18]. Therefore, as suggested by previous research, the ability of gD or gDtr to induce an effective immune response seems to depend on the route of immunization and the type of vector.
Antibodies produced by immunization with the vectors showed no neutralizing activity. This is in agreement with a recent study in which rabbits were immunized with a bovine herpesvirus 4 vector expressing BHV-1 gD ectodomain and bovine viral diarrhoea virus (BVDV) glycoprotein E2 ectodomain [4]. The rabbits in this investigation produced neutralizing antibodies against BVDV but not BHV-1 [4]. This response may also be related to the route of immunization.
In summary, our results suggest that HSV-1-based amplicons could be good candidates for vector vaccines against IBR. However, further studies are needed to identify the best routes of immunization with these vectors. It would also be interesting to evaluate the dose required and cellular responses produced both in mice and cattle, the natural hosts of IBR.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download