Abstract
Monoclonal antibodies (mAbs) specific for the abnormal prion protein isoform (PrPres) are indispensable for diagnosing chronic wasting disease (CWD). In this study, eight mAbs were developed by immunizing PrP knockout mice with recombinant elk PrP and an immunogenic PrP peptide. The reactivity of the mAbs to recombinant PrP and the PrP peptide was measured, and their isotypes were subsequently determined. Among them, four mAbs (B85-05, B85-08, B85-12, and B77-75) were shown by Western blotting to recognize proteinase K-treated brain homogenate derived from an elk suffering from CWD.
Chronic wasting disease (CWD) is a prion disorder that occurs in cervids. CWD was initially observed in captive mule deer and black-tailed deer in Colorado and Wyoming in the USA from 1967 to 1979 [14]. This disease is now widely spread among free-ranging and captive deer as well as elk, especially in the USA and Canada [3]. In recent times, CWD outbreaks have been reported among elk in Korea that were imported from Canada [11]. Clinical signs of CWD include progressive weight loss, excessive salivation, ataxia, and weakness [12]. Additionally, histological lesions such as spongiform changes in the grey matter, intraneuronal vacuolation, and astrocytosis are mainly observed in the central nervous system [15]. CWD is caused by infection with the abnormal isoform of prion protein (PrPres) just like other transmissible spongiform encephalopathies (TSEs). However, unlike most other TSEs, the spreading of CWD is largely mediated by horizontal transmission [7]. CWD-associated PrPres is excreted though the saliva, urine, and feces of infected animals [4]. Therefore, most CWD infections in nature are initiated through oral exposure to PrPres in the environment.
CWD diagnosis is primarily made by immunohistochemistry (IHC) or immunoblot assays to identify the presence of PrPres in appropriate specimens such as central nervous and lymphoid tissues using PrPres-specific monoclonal antibodies (mAbs) [13]. IHC is the gold standard among several assays for diagnosing preclinical cases of CWD [9]. Partial resistance to hydrolysis by proteinase K (PK) is a feature of infectious PrPres [6]. Therefore, three protein bands 22-30 kDa in size are typically observed by Western blotting in specimens containing infectious PrPres after treatment with PK [10]. Since PrP-specific mAbs were initially generated using recombinant bovine PrP, several antibodies have been subsequently produced with highly conserved regions of ovine and bovine PrPs [5]. Because of their specificity for the mostly conserved epitope in cervid PrP, the antibodies generated from ovine and bovine PrP have been routinely used for the diagnosis of CWD [8,13]. Although PrPs are highly conserved among animal species, there are subtle differences in the amino acid sequences. These differences may contribute to interspecies susceptibility to TSEs and the different immunoreactivities of anti-prion antibodies [2]. To date, there have been no reports on the production of mAbs using cervid PrP as an antigen. Therefore, we developed a panel of new mAbs reactive to PrPres specifically isolated from cervids. These antibodies can serve as a valuable reagent for diagnosing of CWD.
Using genomic DNA extracted from elk brain tissue, a mature PrP region, 660 bp of the elk prion gene from 70 bp to 729 bp, was amplified by PCR using the following primers: 5' TGC AAA AAG CGA CCA AAA CC 3' (forward primer) and 5' CAC AGG AGG GGA GGA GAA GAG GAT 3' (reverse primer). The primers were designed based on the DNA sequence of the elk PrP gene (PRNP) (GenBank accession No. AF016228; National Center for Biotechnology Information, USA). The PCR product was cloned into a TOPO TA cloning vector (Invitrogen, USA). The cloned PRNP was amplified by PCR using the following primers: 5' CAT GCA TGC TGC AAA AAG CGA CCA AAA CC 3' (forward primer) and 5' CCC AAG CTT CAC AGG AGG GGA GGA GAA GAG GAT 3' (reverse primer). The underlined sequences in the forward and reverse primers are sites for the restriction endonucleases Sph I and Hind III, respectively. The PCR product (678 bp) was then cloned into a TOPO TA vector, and the plasmid DNA was digested with Sph I and Hind III. The DNA fragment excised by restriction enzyme digestion was ligated into the pQE30 protein expression vector that had been digested with Sph I and Hind III. After selecting a clone containing the elk PRNP, recombinant elk PrP was expressed in E. coli. Purified recombinant elk PrP was finally identified by Western blot analysis using a Prionics-check Western blot kit (Prionics, Switzerland).
To develop mAbs against elk PrP, three types of antigens were used: the recombinant elk PrP produced in this study, a synthetic PrP peptide conjugated to keyhole limpet hemocyanin (KLH) at its carboxyl terminus (aa 93-107 in elk PrP, WGQ GGT HSQ WNK PSK-KLH), and the same peptide lacking KLH. Two PrP knockout C57BL6 mice [Prnp-/- (Nagasaki) mice, kindly provided by Dr. Y. S. Kim, Hallym University, Korea] were intraperitoneally injected with 0.5 mg of the recombinant elk PrP that maintained a disulfide bond configuration in its structure mixed with Freund's complete adjuvant. After 2 weeks, the same amount of protein mixed with Freund's incomplete adjuvant was injected into the mice as the first boosting. For the second boosting, 0.25 mg of the KLH-conjugated PrP peptide mixed with Freund's incomplete adjuvant was injected into the mice. The last boosting was conducted by injecting a mixture of 0.5 mg of the recombinant elk PrP and 0.25 mg of the synthetic PrP peptide lacking KLH mixed with Freund's incomplete adjuvant.
Spleens were removed from the two immunized mice 1 week after the last boosting. Spleen cells were then fused with SP2/0 Ag14 myeloma cells by the polyethylene glycol method in Dulbecco's modified Eagle's medium/hypoxanthine-aminopterin-thymidine supplement medium. mAbs produced from the hybridoma clones were screened by an ELISA to measure their reactivity to the recombinant elk PrP and PrP peptide (aa 93-107 in elk PrP) conjugated with ovalbumin. Reactivity of the selected mAbs to the elk PrPres was then measured by Western blot analysis using brain tissues obtained from a normal healthy elk and CWD-infected elk (kindly provided by Dr. Y. S. Kim, Hallym University, Korea). 1C5 antibody was included in the assay as a positive control [1].
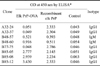
The elk prion gene is composed of a total of 771 bp encoding 256 amino acids. However, mature elk PrP composed of amino acids 24-243 can act as an infectious amyloid precursor (GenBank accession No. AF016227). Therefore, the 660-bp region (70-729 bp) encoding the mature PrP was amplified by PCR. The PCR product was cloned into a cloning vector and sub-cloned into a protein expression vector. Identity of the resulting recombinant elk PrP was verified by SDS-PAGE and Western blot analysis using a PrP-specific antibody. A total of eight clones were selected based on reactivity of the produced antibodies to the PrP peptide and recombinant PrP (Table 1). The reactivity of seven mAbs except for clone A32-24 to both elk normal cellular PrP (PrPC) and elk PrPres was verified by Western blot analysis (Fig. 1). Five mAbs (A32-37, B85-05, B85-08, B85-12, and B77-75) reacted with both PrPC and PrPres from elk brain homogenates that were not treated with PK. However, when the brain homogenates were incubated with PK, only four antibodies (B85-05, B85-08, B85-12, and B77-75) along with the positive control 1C5 antibody recognized PrPres in the homogenate. The data implied that these four mAbs reacted with the PrP 27-30 region resistant to PK treatment. However, the mAb A32-37 did not recognize the PK-resistant region. The epitope recognized by this mAb seemed to be cleaved after exposure to PK. More detailed studies using overlapping peptides are needed to identify the exact epitope recognized by the antibody.
Like other prion diseases, CWD is caused by PrPres accumulation in the central nervous system [12]. Even though PrPC and PrPres have the same amino acid sequences, conformational change results in resistance of PrPres to PK digestion [6]. Therefore, mAbs specific for the PK-resistant PrP 27-30 fragment are absolutely important for developing diagnostic methods to detect PrPres. In the present study, we developed four mAbs reactive to the CWD-associated PrPres by immunizing Prnp-/- mice with recombinant elk PrP. Some previously developed mAbs have limited specificity for only PrP from a specific animal, whereas others demonstrate a broad spectrum of reactivity against PrPs from several species [2]. CWD diagnosis is often conducted using these broadly reactive mAbs generated with PrPs from other animals [8,13]. Since the four mAbs produced in our study were highly reactive to elk PrPres, we expect that these antibodies could be used to develop more reliable methods for diagnosing CWD. In addition, these newly developed mAbs could be help detect human and animal prion diseases such as variant Creutzfeldt-Jakob disease, scrapie, and bovine spongiform encephalopathy.
Figures and Tables
References
1. Choi JK, Park SJ, Jun YC, Oh JM, Jeong BH, Lee HP, Park SN, Carp RI, Kim YS. Generation of monoclonal antibody recognized by the GXXXG motif (glycine zipper) of prion protein. Hybridoma (Larchmt). 2006. 25:271–277.

2. Furuoka H, Yabuzoe A, Horiuchi M, Tagawa Y, Yokoyama T, Yamakawa Y, Shinagawa M, Sata T. Species-specificity of a panel of prion protein antibodies for the immunohistochemical study of animal and human prion diseases. J Comp Pathol. 2007. 136:9–17.

3. Gilch S, Chitoor N, Taguchi Y, Stuart M, Jewell JE, Schätzl HM. Chronic wasting disease. Top Curr Chem. 2011. 305:51–77.

4. Haley NJ, Mathiason CK, Carver S, Zabel M, Telling GC, Hoover EA. Detection of chronic wasting disease prions in salivary, urinary, and intestinal tissues of deer: potential mechanisms of prion shedding and transmission. J Virol. 2011. 85:6309–6318.

5. Korth C, Stierli B, Streit P, Moser M, Schaller O, Fischer R, Schulz-Schaeffer W, Kretzschmar H, Raeber A, Braun U, Ehrensperger F, Hornemann S, Glockshuber R, Riek R, Billeter M, Wüthrich K, Oesch B. Prion (PrPSc)-specific epitope defined by a monoclonal antibody. Nature. 1997. 390:74–77.

6. McKinley MP, Bolton DC, Prusiner SB. A protease-resistant protein is a structural component of the scrapie prion. Cell. 1983. 35:57–62.

7. Miller MW, Williams ES. Prion disease: horizontal prion transmission in mule deer. Nature. 2003. 425:35–36.
8. O'Rourke KI, Baszler TV, Miller JM, Spraker TR, Sadler-Riggleman I, Knowles DP. Monoclonal antibody F89/160.1.5 defines a conserved epitope on the ruminant prion protein. J Clin Microbiol. 1998. 36:1750–1755.
9. O'Rourke KI, Zhuang D, Lyda A, Gomez G, Williams ES, Tuo W, Miller MW. Abundant PrPCWD in tonsil from mule deer with preclinical chronic wasting disease. J Vet Diagn Invest. 2003. 15:320–323.
10. Race RE, Raines A, Baron TGM, Miller MW, Jenny A, Williams ES. Comparison of abnormal prion protein glycoform patterns from transmissible spongiform encephalopathy agent-infected deer, elk, sheep, and cattle. J Virol. 2002. 76:12365–12368.

11. Sohn HJ, Kim JH, Choi KS, Nah JJ, Joo YS, Jean YH, Ahn SW, Kim OK, Kim DY, Balachandran A. A case of chronic wasting disease in an elk imported to Korea from Canada. J Vet Med Sci. 2002. 64:855–858.

12. Spraker TR, Miller MW, Williams ES, Getzy DM, Adrian WJ, Schoonveld GG, Spowart RA, O'Rourke KI, Miller JM, Merz PA. Spongiform encephalopathy in free-ranging mule deer (Odocoileus hemionus), white-tailed deer (Odocoileus virginianus) and Rocky Mountain elk (Cervus elaphus nelsoni) in northcentral Colorado. J Wildl Dis. 1997. 33:1–6.

13. Spraker TR, O'Rourke KI, Balachandran A, Zink RR, Cummings BA, Miller MW, Powers BE. Validation of monoclonal antibody F99/97.6.1 for immunohistochemical staining of brain and tonsil in mule deer (Odocoileus hemionus) with chronic wasting disease. J Vet Diagn Invest. 2002. 14:3–7.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download