Abstract
The feasibility of virtual otoscopy (VO) imaging was evaluated in five dogs with experimentally induced otitis media, two control dogs, and two canine patients with otitis media. VO images of the tympanic cavity and ossicles were generated with commercially available software using raw computed tomography (CT) data. Eight out of 10 ears inoculated with pathogen exhibited obvious clinical signs associated with otitis externa. CT images revealed soft tissue density material occupying the tympanic bulla compatible with otitis media in three dogs with experimentally induced otitis media and two patients. No remarkable features were observed on the radiographs. Four different VO views (ear canal, tympanic bulla, eustachian tube, and ossicular chain) were created. Promontory, cochlea window, tympanic, and septum bulla as well as ossicles were easily and clearly distinguished except for the incus and stapes of the clinical patients. VO images were not more suitable than images created with conventional CT for accurately diagnosing otitis media in this study. However, it appears that VO could be more feasible for assessing the complex structure of the inner ear in dogs with fluid-filled tympanic cavities since fluid accumulation within the tympanic bulla did not affect the evaluation of bony tissue in the middle ear on VO images.
Conventional diagnostic imaging modalities such as radiography [23] and ultrasonography [10,14] used primarily in veterinary medicine cannot reliably identify lesions affecting the middle or inner ear. Today, advanced noninvasive diagnostic techniques such as computed tomography (CT) and magnetic resonance imaging (MRI) are used to evaluate the inner or middle ear [1,4,5,9,13,15,22]. In particular, virtual otoscopy (VO) enables non-invasive 3D endoluminal imaging of the complex inner ear structure. The computer-rendered images allow constant luminal visualization that permits navigation along the inner surfaces in a manner similar to conventional fiberoptic endoscopy. Virtual endoscopy has been studied extensively and was recently used in practice as a tool for radiological diagnosis, surgical planning, and teaching in human medicine [3,16,20,21].
Virtual endoscopy techniques for examining the ear are being developed because the images allow improved visual recognition of the fine temporal bone structures. VO of the middle ear is generally still not used in veterinary medicine. Previously, VO alone was investigated as a means for generating anatomic reference images of the canine middle ear in normal dogs [6]. However, no information on VO images has been reported in veterinary medicine. Therefore, the aim of this study was to evaluate the feasibility of VO through visualization of the tympanic cavity including ossicles in experimentally-induced otitis media and clinical canine patients with fluid accumulation in the tympanic cavity.
Seven healthy male beagles (1 to 4 years old, 7.5 to 10.7 kg; Chemon, Korea) were included in our study. Additionally, we evaluated two canine patients, one Maltese (8 years old, 3.4 kg) and a mixed breed dog (6 years old, 5.8 kg), with otitis media based on clinical features and CT images that revealed hyperatteuated density indicative of fluid- or soft tissue-filled tympanic bulla. The investigation was carried out in accordance with the guidelines of the Animal Care Committee of the College of Veterinary Medicine, Chonbuk National University (Korea).
All of the animals were administrated dexamethasone [1.0 mg per animal via intramuscular injection (Dexason; Daesung, Korea)] 12 h before inoculation. While under general anesthesia with Zoletile (Virbac, France) five of seven healthy beagle dogs were inoculated using a 'mouse syringe' (SamwooKurex, Korea) with a culture of coagulase negative 5 × 108 Staphylococcus aureus-571 (UOP) provided by National Veterinary Research and Quarantine Service (Korea) via the transtympanic membrane bilaterally (a total of ten ears) as previously described [11]. The two remaining healthy dogs were inoculated with the same amount (1.0 mL) of saline solution. After inoculation, clinical signs including indications of ear pain, scratching, head shaking, and cerumen production were observed daily for 3 weeks.
Radiographic examination (lateral and dorsoventral projection) with the conventional X-ray machine (Acoma, Japan) and CT scanning were performed prior to and 15 days after inoculation by multidetector CT scanner (Somatom sensation 16; Simense, Germany). All of the animals were fasted for 24 h prior to CT scanning and premedicated with atropine (Daewon, Korea) before induction of general anesthesia. Radiography and CT studies were performed with the two canine patients. General anesthesia was induced and maintained with propofol (Provive 1%; Claris Lifesciences, India) delivered intravenously.
For CT scanning, the dogs were placed in a sternal recumbent position using a position bag to support the head and cervical spine. Animals with a fluid-filled tympanic cavity were also placed in a dorsal recumbent position. The head was extended with the hard palate parallel to the CT table, and fixed in this position with medical tape (3M Micropore; 3M, USA). Transverse images were obtained with multi-detector-row CT scanners (Somatom Sensation 16 and Somatom Plus 4; Siemens, Germany). The CT scanning parameters were 120 kV, 60 mA, 0.5 sec/rotation, 90-mm field of view with a 1-mm scan slice thickness with no intervening gap, one helical pitch, and a 512/512 matrix size. A total of 40 to 50 axial CT images (window width = 4,000; window level = 400) were obtained per scan sequence. The images were then transferred to a computer equipped with commercially available software (Rapidia; Infinit, Korea) that can produce virtual endoscopic images. Two radiologists (KC Lee and HS Kwak, Chonbuk National University) interpreted the high-resolution CT (HRCT) images as well as the virtual endoscopic images. The threshold values varied from -450 to 1,300 depending on the part of the optimally visualized structures. Bony and/or soft tissue surfaces could be visualized according to the needs of the evaluator. Navigation from the external canal to the tympanic cavity using VO was possible by moving the arrows on the axial CT images in three different planes: transverse, sagittal, and dorsal. Navigation was also possible in the rostral or caudal, dorsal or ventral, and left or right directions and on all sides of middle ear. Identity of the structures visible on the VO images was verified by pointing to a spot in the VO view. The position of this spot was then simultaneously shown on the two-dimensional (2D)-CT images.
The three-dimensional images were reviewed by two experienced radiologists (H.S. Kwak and K.C. Lee, Chonbuk National University). Image quality with respect to the nine anatomic structures examined was scored using the following grading system: 0, anatomical structure not detectable; 1, anatomical structure detected but not clearly defined; and 2, anatomical structure clearly defined.
Eight out of 10 ears inoculated with the bacterial pathogen exhibited obvious clinical signs of infection while the two control dogs did not show any clinical signs of ear disease. CT scanning revealed fluid-filled tympanic cavities in three affected ears of three dogs inoculated with the infectious agent. No remarkable findings were observed on the radiographs in any of the animals. Qualitative measurements of the major inner region structures of all 10 ears are shown on Table 1. Conventional CT imaging revealed soft tissue density occupying the tympanic bulla, indicative of otitis media, in the experimentally infected animals and clinical canine patients (Figs. 1~3).
The head of the malleus, incus, stapes, and vestibular window were distinguishable but not clearly viewed in the experimental dogs. In the clinical patients, most of the structures were not distinguishable except for the malleus. The rostral process of the malleus was barely distinguishable. Internal structures such as the manubrium of the malleus, promontory, cochlea window, and septum bulla were identifiable on the VO images without interference by the fluid inside the bulla cavity (Figs. 4 and 5). Ossicles of the clinical patients, however, were not discernable from one another (Figs. 3 and 6).
Several techniques can be used to facilitate detailed visualization of the middle ear. Indirectly, advanced imaging modalities such as CT and MRI provide useful information for diagnosing and evaluating middle ear problems [15,17] in veterinary medicine. Artifactual effects cause the fluid-filled bulla to appear thicker than that of the normal bulla on axial CT images. However, no correlation between the MR signal intensity of material within the middle ear and final diagnosis have been noted. CT and MR images must therefore be interpreted with caution [2].
Middle ear endoscopy provides an excellent direct view of the surgical micromorphology and pathology of the middle ear [7]. Video otoscopic treatment can also reduce the need for conventional surgery [19]. In humans, complications associated with otoendoscopy may include iatrogenic fracturing of the lenticular process of the incus, non-healing of atrophic thin tympanic membrane, and non-healing of a previous tympanoplasty graft [24].
VO is an alternative method to observe the inside of the middle ear. Although a topographical image produced by VO cannot depict color shades of the canal itself, which can be detected by fiberoptic endoscopy [12,26], VO has been widely investigated in human medicine as a supplement to diagnostic imaging and surgical planning [8,12,18,25]. In veterinary medicine, the utility of VO for examining the inside ear was recently assessed in normal dogs [6]. No study, however, has reported on the application of VO in dogs with an infectious ear disease such as otitis media.
In the present study, VO was used to visualize middle ear structures to assess the fluid-filled tympanic bulla observed in the experimental dogs and clinical patients with otitis media. As reported in a previous VO study in normal dogs, one advantage of VO is that this technique provides more topographical information of the inner ear than 2D-CT [6]. Furthermore, only simple post-processing of CT data is needed to produce a VO image.
The primary focus in this study was visualization of the tympanic cavity including ossicles using VO images. Similar to a previous investigation [6], some important technical adjustments, such as using different viewing angles along with the optimal use of thresholds and color shading that affect the final VO results, were required for our study. Most of the main bony structures in the middle ear could be identified. However, tiny muscles and nerves were not observed on the VO images as expected.
The visibility and clarity of the inside ear including almost all the manubrium of the malleus, promontory, cochlea window, and septum bulla in the view from the tympanic bulla toward the ossicular chain. in our study were similar to previous reported results in normal dogs [6]. For the most part, the structures seen in the normal ear cavity were also visualized in this present study, and the incus and stapes could be seen after threshold adjustment. Interestingly, the promontory, cochlea window, and septum bulla in the middle ear were identifiable regardless of fluid accumulation. As in the normal study with normal dogs, the manubrium as well as the head, neck, and process of the malleus could be easily distinguished, but not in the patient with otitis media. The bony structure of ossicles was not discernable since the ossicles were not clearly observed on the axial CT images for the patient. It is believed that post-processed 3D-CT images, including ones generated by VO, are greatly dependent on raw data from the axial CT image. In addition, the small structure of the ossicles and pathologic changes may contribute to the poor ossicles imaging in the clinical patients. In other words, once the Hounsfield number is perceptibly different between the structures, VO would be available to visualize the interest regions in any circumstances. Visualization of the inside structure like ossicles through threshold adjustment in VO was not interfered by soft tissue and fluid density within the tympanic bulla. Meanwhile, most of the fluid should be removed to assure a clear scope path that facilitates unobstructed visualization of the canal with fiberoptic otoscopy.
This study had a couple limitations. First, a small number of experimental dogs with a few affected ears were involved in our investigation. Additionally, these animals did not have any remarkable CT features of otitis media (such as sclerosis along with thickening and possible lysis of the tympanic bulla) except for fluid-filled tympanic cavities. Finally, only two canine patients were included in this study that were examined with a different CT machine which inherently provided slightly different spatial resolution.
Although VO did not provide enough data to make a definitive diagnosis of middle ear problems, this technique could be used to evaluate cases with fluid-filled tympanic cavities frequently observed with ear disease such as otitis media. Our findings also demonstrated that VO can be consistently used to obtain information about anatomical structures in the middle ear. Further studies should be conducted with various breeds of dogs, including small-sized animals, with a variety of ear diseases such as natural occurring otitis media, interna, polyps, and aggressive infiltrative disease.
Figures and Tables
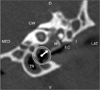 | Fig. 1Transverse computed tomography (CT) image of the temporal bone with the dog in a dorsal recumbent position. Fluid opacity was seen around the tympanic bulla. The arrow within the circle shows the virtual otoscopy (VO) direction of Fig. 4. EC: ear canal, TB: tympanic bulla, M: malleus, I: incus, CW: cochlear window, D: dorsal, V: ventral, MED: medial, LAT: lateral. |
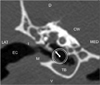 | Fig. 2Transverse CT image of the temporal bone with the dog in a ventral recumbent position. Fluid level in the ventral aspect was observed in the tympanic bulla. The arrow within the circle shows the VO direction of Fig. 5. |
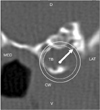 | Fig. 3Transverse CT image of the temporal bone in a canine patient with otitis media placed in a ventral recumbent position. A fluid-filled tympanic bulla and an absence of air density in the ear canal were observed. The arrow within the circle shows the VO direction of Fig. 6. |
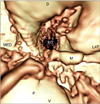 | Fig. 4VO view from the tympanic bulla toward the auditory tube. Fluid opacity did not interfere with identifying the internal structures of the middle ear. S: stapes, P: promontory. |
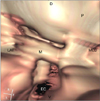 | Fig. 6VO view from the tympanic bulla toward the auditory tube in the same dog shown in Fig. 5. |
References
1. Allgoewer I, Lucas S, Schmitz SA. Magnetic resonance imaging of the normal and diseased feline middle ear. Vet Radiol Ultrasound. 2000. 41:413–418.

2. Barthez PY, Koblik PD, Hornof WJ, Wisner ER, Seibert JA. Apparent wall thickening in fluid filled versus air filled tympanic bulla in computed tomography. Vet Radiol Ultrasound. 1996. 37:95–98.

3. Boor S, Maurer J, Mann W, Stoeter P. Virtual endoscopy of the inner ear and the auditory canal. Neuroradiology. 2000. 42:543–547.

4. Cole LK, Samii VF. Contrast-enhanced computed tomographic imaging of the auditory tube in mesaticephalic dogs. Vet Radiol Ultrasound. 2007. 48:125–128.

5. Dvir E, Kirberger RM, Terblanche AG. Magnetic resonance imaging of otitis media in a dog. Vet Radiol Ultrasound. 2000. 41:46–49.

6. Eom K, Kwak H, Kang H, Park S, Lee H, Kang H, Kwon J, Kim I, Kim N, Lee K. Virtual CT otoscopy of the middle ear and ossicles in dogs. Vet Radiol Ultrasound. 2008. 49:545–550.

7. Fabinyi B, Klug C. A minimally invasive technique for endoscopic middle ear surgery. Eur Arch Otorhinolaryngol. 1997. 254:Suppl 1. S53–S54.

8. Frankenthaler R, Moharir V, Kikinis R, van Kipshagen P, Jolesz F, Umans C, Fried MP. Virtual otoscopy. Otolaryngol Clin North Am. 1998. 31:383–392.

9. Garosi LS, Dennis R, Schwarz T. Review of diagnostic imaging of ear diseases in the dog and cat. Vet Radiol Ultrasound. 2003. 44:137–146.

10. Griffiths LG, Sullivan M, O'Neill T, Reid SWJ. Ultrasonography versus radiography for detection of fluid in the canine tympanic bulla. Vet Radiol Ultrasound. 2003. 44:210–213.

11. Imori T, Matsuda H, Tohjo M, Kamata Y. An experimental production of suppurative otitis media in dog, and a trial to evaluate the therapeutic effect of cefmetazole on this otitis media. Jpn J Antibiot. 1982. 35:2277–2287.
12. Karhuketo TS, Dastidar PS, Ryymin PS, Laasonen EM, Puhakka HJ. Virtual endoscopy imaging of the middle ear cavity and ossicles. Eur Arch Otorhinolaryngol. 2002. 259:77–83.

13. Kneissl S, Probst A, Konar M. Low-field magnetic resonance imaging of the canine middle and inner ear. Vet Radiol Ultrasound. 2004. 45:520–522.

14. Lee J, Eom K, Seong Y, Lee H, Park J, Lee J, Jang K, Lee K, Oh T, Lee S, Yoon J, Lee H, Choi H, Lee Y, Chang D. Ultrasonographic evaluation of the external ear canal and tympanic membrane in dogs. Vet Radiol Ultrasound. 2006. 47:94–98.

15. Love NE, Kramer RW, Spodnick GJ, Thrall DE. Radiographic and computed tomographic evaluation of otitis media in the dog. Vet Radiol Ultrasound. 1995. 36:375–379.

16. Neri E, Caramella D, Panconi M, Berrettini S, Sellari Franceschini S, Forli F, Bartolozzi C. Virtual endoscopy of the middle ear. Eur Radiol. 2001. 11:41–49.

17. Owen MC, Lamb CR, Lu D, Targett MP. Material in the middle ear of dogs having magnetic resonance imaging for investigation of neurologic signs. Vet Radiol Ultrasound. 2004. 45:149–155.

18. Pandey AK, Bapuraj JR, Gupta AK, Khandelwal N. Is there a role for virtual otoscopy in the preoperative assessment of the ossicular chain in chronic suppurative otitis media? Comparison of HRCT and virtual otoscopy with surgical findings. Eur Radiol. 2009. 19:1408–1416.

19. Rawlings CA. Diagnostic rigid endoscopy: otoscopy, rhinoscopy, and cystoscopy. Vet Clin North Am Small Anim Pract. 2009. 39:849–868.

20. Rodt T, Bartling S, Schmidt AM, Weber BP, Lenarz T, Becker H. Virtual endoscopy of the middle ear: experimental and clinical results of a standardised approach using multi-slice helical computed tomography. Eur Radiol. 2002. 12:1684–1692.

21. Rodt T, Ratiu P, Becker H, Bartling S, Kacher DF, Anderson M, Jolesz FA, Kikinis R. 3D visualisation of the middle ear and adjacent structures using reconstructed multi-slice CT datasets, correlating 3D images and virtual endoscopy to the 2D cross-sectional images. Neuroradiology. 2002. 44:783–790.

22. Rohleder JJ, Jones JC, Duncan RB, Larson MM, Waldron DL, Tromblee T. Comparative performance of radiography and computed tomography in the diagnosis of middle ear disease in 31 dogs. Vet Radiol Ultrasound. 2006. 47:45–52.

23. Shell LG. Otitis media and otitis interna. Etiology, diagnosis, and medical management. Vet Clin North Am Small Anim Pract. 1988. 18:885–899.
24. Silverstein H, Jackson LE. Wiet RJ, editor. Office-based minor surgery: otoendoscopy and inner ear perfusion. Ear and Temporal Bone Surgery: Minimizing Risks and Complications. 2006. 1st ed. New York: Thieme Medical Publishers;275–284.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download