Introduction
Heat shock proteins (HSPs) or stress proteins, which play an essential role in the maintenance of cellular homeostasis, are the products of several distinct gene families produced under both physiological and stress conditions [2]. Alpha basic-crystallin (αB-c) belongs to a family of small HSPs, and is expressed in response to different stresses including heat shock, oxidative stress, metal ions, and cytokines [6,11]. αB-c is constitutively expressed in many humans and animals tissues such as the lens of the eyes, heart, and skeletal muscle [9,16]. αB-c has chaperone-like properties which prevent the aggregation of damaged or proteins misfolded due to cell stress, and has been shown to inhibit apoptosis [10].
Mammary tumors, the most common neoplasms in female dogs, may provide a useful model for human breast cancer research [15]. Both benign and malignant neoplasms account for approximately 50% of all tumors in dogs and show wide pathological and clinical heterogeneity [12,18]. After surgical intervention, approximately 48% of the affected dogs die or are euthanized within a 1-year period because of tumor recurrence or metastasis [7]. Therefore, additional and reliable prognostic tools are required for effective risk assessment. The aims of this study were to evaluate the prognostic and/or diagnostic factors associated with canine mammary tumors in dogs by analyzing the immunohistochemical expression of αB-c.
Materials and Methods
Formalin-fixed, paraffin-embedded blocks containing 51 naturally occurring canine mammary tumors (11 benign and 40 malignant) were selected retrospectively and acquired from the Veterinary Laboratory (Italy) and Department of Pathology, Faculty of Veterinary Medicine, Ondokuz Mayis University (Turkey). Additionally, eight normal canine mammary glands obtained from the dogs that had died of causes unrelated to tumor development served as healthy control tissues. Three pathologists (TG, MYG and MS) independently diagnosed the tumors according to the World Health Organization classifications for canine mammary neoplasm [13].
Sections from all the tissue samples were cut (at a thickness of 4 µm) and stained with haematoxylin and eosin. Additional sections were placed on slides coated with 3-aminopropyltriethoxysilane (Sigma, USA) and stained using a streptavidin-biotin-peroxidase complex technique (Histostain Plus kit; Zymed, USA) with a monoclonal anti-αB-c antibody (1/1,000 dilution, SPA-222; Stressgen, Canada). Incubation with amino ethyl carbazole (AEC substrate kit; Invitrogen, USA) or 3,3'-diaminobenzidine (DAB chromogen/substrate kit; Scytek, USA) as a chromogen in H2O2 was performed for 10 min. The sections were counterstained with Mayer's hematoxylin for 1 min, rinsed with tap water, and mounted with an aqueous mounting medium (Vision Mount; Lab Vision, USA).
The percentages of the total area of the immunohistochemically positive cells were assessed with a microscopy image analysis system (Bs200P; BAB Software, Turkey). A total of 10 high-power fields were randomly chosen and analyzed at × 400. The findings were categorized as follows: (0) no positively stained tumor cells; (1) 5~25%; (2) 26~50%; and (3) greater than 51% positive tumor cells. Statistical analysis was carried out using a logistic regression analysis. The results were considered statistically significant if p < 0.05.
Results
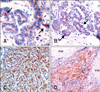
The immunohistochemical results of αB-c expression and histopathologic diagnoses are summarized in Table 1. In the control mammary tissues, a few luminal epithelial cells were positive but the basal myoepithelial cells were negative (Fig. 1A). Muscles (e.g., M. supramammarius) were always positive and were used as positive internal controls when present.
In benign or malignant simple type tumors, αB-c expression was observed in the luminal epithelial cells (Fig. 1B) while the myoepithelial cells were negative. Additionally, αB-c immunoreactivity of the epithelial cells in malignant tumors was more intense than that observed in the benign type. In both benign and malignant complex type tumors, positive staining was noted predominantly in the cytoplasm of the neoplastic epithelial cells (Fig. 1C). αB-c immunoreactivity was also found in neoplastic myoepithelial cells. However, αB-c-positive mesenchymal elements of the carcinosarcoma, such as cartilage or bone, were not observed (Fig. 1D).
The numbers of αB-c positive cells were statistically significant different between the control canine mammary tissues and benign tumors (p < 0.05). Statistically, significant differences of αB-c immunopositive cells were also found in between benign and malignant tumors (p < 0.05). Additionally, the most significant difference of immunopositive cell count was observed between normal mammary gland tissues and malignant tumors (p < 0.001).
Discussion
The results of the current study indicates that the number of αB-c-positive cells were increased in benign and malignant canine mammary tumors when compared to control mammary gland, and the number of αB-c-positive cells were higher in malignant tumors when compared with benign tumors.
αB-c is a member of the mammalian small HSP superfamily [8]. This factor is expressed in many tissues and organs, and acts as a molecular chaperon. It is upregulated by physiologic stress and neurodegenerative diseases [1,5,8,16]. Additionally, αB-c is expressed in breast and the other cancers, such as gliomas, and prostate and renal cell carcinomas, in humans [3,4,14,19]. However, the best of our knowledge, there is no such study performed in animals and the present report documents a connection between αB-c and mammary tumors in canines.
In the normal human breast tissue, αB-c is predominantly expressed in myoepithelial cell [14]. In contrast to this report, in the current study, very low level expression of αB-c was observed in the luminal epithelial cells of normal canine mammary tissues, but αB-c immunepositive reactivity in the myoepithelial cells was not observed. It is possible that this difference might be species-specific or related to the physiological stage of the control mammary gland tissues. Sitterding et al. [17] reported that αB-c may be used as a myoepithelial marker, but it does not have any special advantage over currently used markers such as smooth muscle myosine. In contrast, αB-c immune reactivity in the myoepithelial cells was not observed in our study. Therefore, αB-c immunostaining in the canine mammary gland tissues could not be suggested as a myoepithelial marker.
Chelouche-Lev et al. [3] have reported that out of 672 human breast tumors, 608 (90%) are positive for αB-c immunostaining. In contrast, Moyano et al. [14] noted 39 positively immunostained samples out of 361 (11%) human breast cancer cases. Sitterding et al. [17] reported that αB-c expression is observed in human basal-like (81%) and metaplastic (86%) breast cancer. In the present study, the rate of αB-c expression in canine mammary gland tumors was 88.2% (45 out of 51). Although it is difficult to compare canines to humans, our data are similar to the findings of Chelouche-Lev et al. [3] and Sitterding et al. [17], but different from those of Moyano et al. [14].
Differences in the number of αB-c-immunolabelled cells were found to be statistically significant between the normal canine mammary tissues and the benign and malignant tumors. These differences were thought to be evidence that αB-c was involved in oncogenesis because of the anti-apoptotic effect of αB-c. This effect probably involves the suppression of caspase-3 activation and/or preventing the mitochondrial translocation of pro-apoptotic Bcl-2 family members such as Bax [10].
In conclusion, we believe that αB-c can play a role in the carcinogenesis of canine mammary glands because of the increased immunoreactivity of αB-c we observed in neoplastic tissues compared to the normal canine mammary gland tissues. αB-c has an anti-apoptotic effect and this anti-apoptotic effect can be important in chemotherapy resistance of the cancer. Additionally, targeted inhibition of αB-c may be a new strategy to the cancer therapy.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download