Abstract
In this study, we developed a novel tool for purifying two mycotoxins, aflatoxin B1 (AFB1) and zearalenone (ZEN), in feed. This system utilized monoclonal antibodies (mAbs) against AFB1 and ZEN, and magnetic nanoparticles (MNPs). Among ten MNPs with different diameters and functional groups, a 100-nm diameter MNP (fMA) conjugated to an amine group (-NH2) was found to be optimum for coupling with mAbs. The optimal mAb concentrations for coupling to the fMA along with mycotoxin purification capacities of the fMA-mAb conjugates (fMA-AFB1 and fMA-ZEN) were determined. A comparison of mean recovery rates (from corn and product X feed) between the fMA-mAb conjugates and immunoaffinity columns (IAC-AFB1 and IAC-ZEN) showed that the rate for fMA-AFB1 (90~92% and 81~88%) was higher (p > 0.05) than that of IAC-AFB1 (81~84% and 72~78%) for AFB1 (5, 10, 15 ng/mL), and the rate for fMA-ZEN (99~100% and 92~94%) was significantly higher (p < 0.01) than that of IAC-ZEN (86~88% and 81~88%) for ZEN (10, 25, 50 ng/mL) except at a concentration of 10 ng/mL, demonstrating the remarkable purification efficiency of the novel fMA-mAb method. Additionally, mycotoxin purification was much faster using our novel method (approx. 5 min) than the IAC-based technique (> 30 min). This study suggests that the novel purification system we developed would be a useful tool for monitoring and regulating mycotoxin contamination in feed, and replace IAC methods.
Mycotoxins are small compounds produced by fungi with specific moisture and temperatures, and pose a threat to animal and human health [8,19]. Zearalenone (ZEN) is produced by Fusarium (F.) fungi including F. graminearum, F. culmorum, F. equiseti, F. cerealis, F. crookwellense, and F. semiectum [2]. This toxin is heat-stable and found in maize, barley, oats, wheat, rice, and bread. ZEN is a potent estrogenic metabolite which causes reproductive problems, such as infertility and abortion [10], in animals that consume cereals contaminated with the toxin. The most susceptible animal is swine [13]. Aflatoxin is produced by Aspergillus (A.) flavus and A. parasiticus, and exists as four major types: B1, B2, G1, and G2 [14,23]. Hazardous effects of this toxin have been well documented. In particular, aflatoxin B1 (AFB1) is an extremely potent liver carcinogen [14].
Detection and surveillance of mycotoxins is highly important for preventing animals and/or humans from consuming grain products contaminated with these compounds. Recent regulations regarding AFB1 and ZEN were implemented to minimize health problems caused by the toxins although the maximum permissible toxin levels differ among countries [24]. Several methods have been employed to monitor toxin levels in grain products including thin-layer chromatography [11], gas chromatography.mass spectrometry [16], high-performance liquid chromatography (HPLC) [11,17,20], and enzyme-linked immunosorbent assays [11,18]. When identifying official methods for monitoring purposes, purification efficacy for mycotoxin testing is critical because it ensures accuracy and precision when determining the level of mycotoxin contamination. The use of immunoaffinity columns (IACs) with monoclonal antibodies (mAbs) is currently the most popular method for purifying mycotoxin contaminants from samples [3,17,20]. However, this method is expensive, time-consuming, and produces inconsistent results, which makes it impractical.
Nanoparticles are substances that have recently been used for various applications such as the treatment of disease, drug delivery, and diagnostics [4,9,15]. Recent studies have indicated the usefulness of nanoparticles for recovering heavy metals from water and isolating harmful microbes from livestock products [6,7,21]. The purpose of the current study was to develop an advanced purification tool for isolating mycotoxins by utilizing mAbs and magnetic nanoparticles (MNPs). We hypothesized that this technique would facilitate purification by magnetism and combine high capacity for purification with fast dispersibility due to the small size of the particles.
mAbs (kj-AFB against AFB1 and kk-ZEN against ZEN) were produced in our laboratory and shown to have greater specificity and affinity than commercial mAbs (Fig. 1). MNPs (fluidMAG-MNPs) with five different functional groups [fluidMAG-amine (fMA), fluidMAG-carboxyl, (fMC), fluidMAG-protein A/G (fMA/fMG), fluidMAG-streptavidin (fMS)] used in this study were purchased from Chemicell (Germany; Table 1). fluidMAG-MNPs are ferrofluids consisting of an aqueous dispersion of magnetic iron oxides (Fe2O3) with diameters of 100 nm and 200 nm. The particles are covered with hydrophilic polymers that protect against aggregation promoted by foreign ions. Terminal functional groups such as ion-exchange groups or reactive groups for covalent immobilization can be used for binding to biomolecules.
The five types of MNPs were activated before being coupled to mAbs following the manufacturer's protocols. Briefly, 2 mg of an fMA suspension was washed three times using a magnet in coupling buffer (0.01 M pyridine, pH 6.0). A glutaraldehyde solution (5%, 1 mL) was added and incubated with the MNPs at room temperature for 30 min. The particles were washed with wash buffer (0.01 M Tris, 0.15 M NaCl; and 0.1% BSA, pH 7.4) by magnetic separation (Fig. 2). mAb coupling was conducted by dissolving 50, 100, and 200 µg of each mAb (kj-AFB or kk-ZEN) in 550 µL of coupling buffer and incubated with the activated magnetic particles for 16 to 24 h at room temperature (Fig. 2). The MNP.mAb conjugates (fMA-AFB1 and fMA-ZEN) were washed and stored in the wash buffer at 4℃ before use.
fMC particles were prepared by adding 2 mg of particle suspension to 1-cyclohexyl-3-(2-morpholinoethyl) carbodiimide metho-p-toluenesulfonate (CMC) and incubating at room temperature. After incubation, the particles were washed with distilled and deionized water. The particles were then incubated with 100 µg of mAb diluted in phosphate buffered saline (PBS). The MNP-mAb conjugates (fMC-AFB1 and fMC-ZEN) were maintained in storage buffer (PBS, 0.1% BSA, and 0.05% sodium azide) at 4℃.
The fMA/fMG particles were prepared by resuspending 2 mg of the MNP suspension in binding buffer (0.1 M sodium phosphate and 0.15 M NaCl, pH 7.5) and washing by magnetic separation. The washed MNPs were coupled with mAbs diluted in PBS at room temperature. The mAb-conjugated MNPs (fMA/fMG-AFB1 and fMA/fMG-ZEN) were stored at 4℃ in PBS containing 0.02% sodium azide.
fMS particles were prepared by washing 2 mg of the particle suspension with PBS by magnetic separation. The particles were coupled with biotinylated mAbs at room temperature. The mAb-coupled MNPs (fMS-AFB1 and fMS-ZEN) were then washed with PBS and stored at 4℃.
Five g of feed samples [corn and mixed grains (product X) without detectable level of mycotoxins in our test condition] were purchased from a feed company in Korea. The feed samples were ground and spiked with 10 ppb AFB1 and 50 ppb ZEN. After vigorous shaking, mycotoxins were extracted by incubation for 3 min in 70% methanol. The extracted samples were passed through Whatman No. 1 filter paper; 10 mL of the filtrate was mixed with 40 mL of PBS (pH 7.4) and used for mycotoxin purification trials in this study.
To purify the mycotoxins, MNP-mAb conjugates were mixed with 5 mL of the processed samples (50 mL) or 5 mL PBS containing mycotoxins for 5 min at room temperature with shaking. After the reaction, MNP-mAb conjugates bound to the mycotoxins were magnetically removed from the supernatant, which was discarded. The mycotoxins were dissociated from the MNP-mAbs by adding 500 µL of 100% methanol and gently shaking. After dissociation, the MNP-mAb particles were magnetically removed perpendicular to gravity, and mycotoxin concentrations in the samples were measured.
IAC-AFB1 (NeoColumn; Neogen, USA) and IAC-ZEN (Easi-extract zearalenone; R-Biopharm, Germany) were used to isolate AFB1 and ZEN, respectively, and the mycotoxins were purified following the manufacturer's protocol. Briefly, the columns were washed with 20 mL PBS by gravity at a flow rate of 5 mL/min. Next, 50 mL of the extracted samples were passed through the washed columns by gravity. The mycotoxins were eluted with 100% methanol and used for HPLC analysis.
Mycotoxins purified by MNP-mAb conjugates or IACs were analyzed by HPLC using a Symmetry C18 column (Waters, USA) for ZEN and an X teorra RP18 column (Waters, USA) for AFB1. The analytical column was equilibrated with water-acetonitrile (50 : 50, v : v) at a flow rate 1 mL/min. The eluted sample (10 µL) was injected into the HPLC system (Waters, USA). Using a fluorescence detector (Waters, USA), sample analysis was conducted at an excitation wavelength of 274 nm and emission wavelength of 440 nm for ZEN, and 365 nm (excitation) and 480 nm (emission) for AFB1.
Ten MNPs (diameters of 100 nm and 200 nm) with five different functional groups were chosen and coupling efficiency with mAbs (kk-ZEN) against ZEN was tested. As shown in Fig. 3, 100 µg of mAb were coupled with 2 mg of MNPs (100-nm diameter). It was found that an average of 96.7% and 63.3% of mAbs were bound to fMA and fMS, respectively. In contrast, less than 8% of the mAbs were bound to the MNPs conjugated to the other functional groups (fMC and fMA/fMG). There was no significant effect associated with MNP size because particles with a 200-nm diameter produced similar results (data not shown). fMA particles with a 100-nm diameter were therefore used for the subsequent studies.
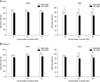
Two mg of fMA particles were incubated with mAbs at three different concentrations (50, 100, and 200 µg) in PBS to determine the optimal concentration of mAbs for MNP coupling. The mycotoxin purification capacity of the MNP-mAb conjugates (fMA-AFB1 and fMA-ZEN) produced with each mAb concentration was also evaluated by incubating 500 µg of the conjugates with 10 ng AFB1 and 50 ng ZEN in PBS, respectively. It was found that kj-AFB1 was increasingly coupled to fMA in a mAb concentration-dependent manner: 17.03 ± 0.76 µg (50 µg), 42.31 ± 2.28 µg (100 µg), and 94.30 ± 3.78 µg (200 µg) per 1 mg of MNPs. However, the purification capacity of fMA-AFB1 declined in a manner inversely proportional to the amount of mAb bound to the MNP: 1.21 ± 0.03 ng (50 µg), 1.07 ± 0.13 ng (100 µg), 0.63 ± 0.09 ng (200 µg) per 1 µg of mAb on fMA-AFB1 complex. A similar observation was made in the coupling test evaluating kk-ZEN coupled to fMA particles: 15.27 ± 0.32 µg (50 µg), 40.25 ± 0.73 µg (100 µg), 96.66 ± 2.96 µg (200 µg) per 1 mg of MNP. In contrast, the ZEN purification capacity declined sharply: 2.56 ± 0.11 ng (50 µg), 2.26 ± 0.08 ng (100 µg), 1.76 ± 0.09 ng (200 µg) per 1 µg of mAb on the fMA-ZEN complex (Fig. 4).
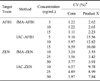
The limit of detection and maximum quantities of mycotoxins purified using fMA-AFB1 (1 mg) or fMA-ZEN (1 mg) were measured with 2 mg fMA and 100 µg mAb (0.1 ng/mL and 45.3 ng/mL for AFB1, respectively, and 2 ng/mL and 90.9 ng/mL for ZEN, respectively). Thus, fMA-AFB1 (1 mg) and fMA-ZEN (1 mg) were used to evaluate recovery rates and comparison with the IAC recovery rates. The average recovery rates of the fMA-AFB1 and fMA-ZEN conjugates were compared with those of IAC-AFB1 and IAC-ZEN by testing corn and product X feed samples spiked with AFB1 (5, 10, and 15 ng) or ZEN (10, 25, and 50 ng). As shown in Fig. 5, recovery rates for fMA-AFB1 (90~92% and 81~88%) were higher (p > 0.05) than those for IAC-AFB1 (81~84% and 72~78%), while the rates for fMA-ZEN (99~100% and 92~94%) were significantly (p < 0.01) higher than those of IAC-ZEN (86~88% and 81~88%) except for 10 ng/mL ZEN in product X. The coefficients of variation (CV) for fMA-AFB1 and IAC-AFB1 associated with the three different mycotoxin concentrations were 1.11~1.22% and 5.97~9.16% for corn feed, respectively, and 2.23~2.65% and 10.6~15.56% for product X, respectively. On the other hand, the CV for fMA-ZEN and IAC-ZEN were 3.24~3.77% and 4.89~6.57% for corn, respectively, and 3.42~3.93% and 7.84~9.78% for product X, respectively (Table 2).
This study is the first report of an animal feed mycotoxin purification system using MNPs. The use of nanoparticles for purifying target molecules such as heavy metals [22] and bacteria [5] has been explored because of particle dispersibility and cost effectiveness of this procedure. Thus, feasible applications in new areas have been discovered. Mycotoxins are a threat to public health because these compounds contaminate cereals and a variety of grains consumed by humans and animals [12]. The current study explored the possible use of MNPs for mycotoxin purification by coupling the particles to mAbs specific for mycotoxins. This was done to find a suitable replacement for current purification methods.
The mAb coupling efficiencies of commercially produced MNPs (100-nm and 200-nm diameters) were tested. The functional groups on the MNPs were capable of binding mAbs through diverse mechanisms. For example, an amine group covalently binds to the carboxyl group of the mAbs whereas the carboxyl group covalently binds to the mAb amine group. Protein A/G binds the Fc portion of mAbs, and streptavidin is specific for biotin molecules on biotinylated mAbs. In our selection tests, MNPs with an amine functional group were found to be the most appropriate for the current study.
The selected MNP (fMA) was used to test MNP-antibody coupling capacity, and mycotoxin purification capacity of the MNP-mAb conjugates was evaluated. Antibodies coupled with the MNPs in an antibody concentration-dependent manner. However, the mycotoxin purification capacity declined as the amount of antibody coupled to the MNPs increased. The surface of nanoparticles has a limited area available for coupling, so it is possible that excess antibody [1] on the MNP surface (7.5 nm in length) might hamper the antibody-antigen reaction due to steric hindrance.
IAC-based techniques are currently the most popular method for purifying mycotoxins from feeds [11,16]. However, this method is expensive, time-consuming, and requires the application of a large volume of solvent, which makes it a tedious process. In the present study, mycotoxin purification efficiency and procedure feasibility of the MNP-mAb conjugates was compared with that of IACs. Each commercial IAC was manufactured to purify more than 100 ng of mycotoxin while the MNP-mAb conjugates (fMA-AFB1 and fMA-ZEN) had the capacity to purify approximately 45.3 ng of AFB1 or 90.9 ng of ZEN. Comparison of the two methods showed that the MNP-mAb conjugates had a significantly (p < 0.01) higher recovery rate and lower CV values compared to the IACs. There could be two possible reasons for the observation. First, the mAbs used in our study were made in the laboratory, and had a demonstrably higher affinity and specificity for toxins compared to commercially available antibodies (data not shown). Second, prolonged washing with large amounts of washing buffer during the IAC procedure might result in a loss of toxins bound to the antibody in the column. Our new method used MNP-mAb conjugates that separate toxins from unbound materials using magnetism. The washing process is also very fast, simple, and uses only a small amount of buffer. Furthermore, our novel method required only approximately 5 min to complete (from antibody binding to elution) whereas the IAC-based procedure took longer than 30 min. More importantly, the amount of mycotoxin purified using our method was more consistent and reproducible compared to the IACs as indicated by the lower CV values (CV value of the MNP-mAb conjugates was less than 4.0 in contrast to 4.8 for the IACs), suggesting that our novel method is more reliable than IACs.
In summary, we developed a new mycotoxin purification method using nanoparticles that exhibited remarkable efficiency. Our findings suggested that this method could replace IAC techniques that are widely used for separating mycotoxins from feed.
Figures and Tables
Fig. 1
Competitive ELISA for measuring mAb specificity and affinity of (A) kj-AFB and (B) kk-ZEN. The mAbs we developed (kj-AFB or kk-ZEN) or commercial mAbs [C] at different dilutions (1/250, 1/500, and 1/1,000 for C; 1/64, 1/128, 1/256, 1/512, and 1/1024 for kk-ZEN; 1/16, 1/32, 1/64, 1/128, and 1/256 for kj-AFB). kj-AFB and kk-ZEN were found to have greater specificity as well as affinity than the commercial mAbs.

Fig. 3
Coupling efficiency of MNPs with a diameter of 100 nm. MNPs (2 mg) were coupled with 100 µg of the kk-ZEN mAb specific for ZEN (n = 3). The amount of bound mAb is presented as the mean ± SE. *p < 0.01.

Fig. 4
Optimal coupling conditions with different concentration of mAbs and the mycotoxin purification capacity. MNPs (2 mg) were coupled with 50, 100, or 200 µg of mAbs (n = 3) and 1 mg each fluidMAG-amine (fMA)-AFB1 (A) and fMA-ZEN (B). All values were significantly different (p < 0.01) except for differences in the amounts of mycotoxin purified by MNPs coupled with 50 µg or 100 µg of AFB1-specific mAb. *Indicates the mean ± SE.

Acknowledgments
This work was funded by the Animal, Plant and Fisheries Quarantine and Inspection Agency, Ministry for Food, Agriculture, Forestry and Fisheries, Korea.
References
1. Ban C, Ramakrishnan B, Sundaralingam M. Crystal structure of the highly distorted chimeric decamer r(C)d(CGGCGCCG)r(G)·spermine complex--spermine binding to phosphate only and minor groove tertiary base-pairing. Nucleic Acids Res. 1994. 22:5466–5476.

2. Caldwell RW, Tuite J, Stob M, Baldwin R. Zearalenone production by Fusarium species. Appl Microbiol. 1970. 20:31–34.
3. Castegnaro M, Tozlovanu M, Wild C, Molinié A, Sylla A, Pfohl-Leszkowicz A. Advantages and drawbacks of immunoaffinity columns in analysis of mycotoxins in food. Mol Nutr Food Res. 2006. 50:480–487.

4. Cuenca AG, Jiang H, Hochwald SN, Delano M, Cance WG, Grobmyer SR. Emerging implications of nanotechnology on cancer diagnostics and therapeutics. Cancer. 2006. 107:459–466.

5. Duan HL, Shen ZQ, Wang XW, Chao FH, Li JW. Preparation of immunomagnetic iron-dextran nanoparticles and application in rapid isolation of E. coli O157:H7 from foods. World J Gastroenterol. 2005. 11:3660–3664.

6. Gao M, Deng C, Fan Z, Yao N, Xu X, Yang P, Zhang X. A simple pathway to the synthesis of magnetic nanoparticles with immobilized metal ions for the fast removal of microcystins in water. Small. 2007. 3:1714–1717.

7. Hibi K, Mitsubayashi K, Fukuda H, Ushio H, Hayashi T, Ren H, Endo H. Rapid direct determination using combined separation by prepared immunomagnetic and flow cytometry of Flavobacterium psychrophilum. Biosens Bioelectron. 2007. 22:1916–1919.

8. Hussein HS, Brasel JM. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology. 2001. 167:101–134.

9. Jain KK. Applications of nanobiotechnology in clinical diagnostics. Clin Chem. 2007. 53:2002–2009.

10. Kallela K, Ettala E. The oestrogenic Fusarium toxin (zearalenone) in hay as a cause of early abortions in the cow. Nord Vet Med. 1984. 36:305–309.
11. Krska R, Schubert-Ullrich P, Molinelli A, Sulyok M, MacDonald S, Crews C. Mycotoxin analysis: an update. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2008. 25:152–163.

12. Lugauskas A, Raila A, Zvicevičius E, Railienė M, Novošinskas H. Factors determining accumulation of mycotoxin producers in cereal grain during harvesting. Ann Agric Environ Med. 2007. 14:173–186.
13. Malekinejad H, Schoevers EJ, Daemen IJJM, Zijlstra C, Colenbrander B, Fink-Gremmels J, Roelen BAJ. Exposure of oocytes to the Fusarium toxins zearalenone and deoxynivalenol causes aneuploidy and abnormal embryo development in pigs. Biol Reprod. 2007. 77:840–847.

14. Martins ML, Martins HM, Bernardo F. Aflatoxins in spices marketed in Portugal. Food Addit Contam. 2001. 18:315–319.

15. McBain SC, Yiu HHP, Dobson J. Magnetic nanoparticles for gene and drug delivery. Int J Nanomedicine. 2008. 3:169–180.
16. Ryu JC, Yang JS, Song YS, Kwon OS, Park J, Chang IM. Survey of natural occurrence of trichothecene mycotoxins and zearalenone in Korean cereals harvested in 1992 using gas chromatography/mass spectrometry. Food Addit Contam. 1996. 13:333–341.

17. Solfrizzo M, De Girolamo A, Visconti A. Determination of fumonisins B1 and B2 in cornflakes by high performance liquid chromatography and immunoaffinity clean-up. Food Addit Contam. 2001. 18:227–235.

18. Thongrussamee T, Kuzmina NS, Shim WB, Jiratpong T, Eremin SA, Intrasook J, Chung DH. Monoclonal-based enzyme-linked immunosorbent assay for the detection of zearalenone in cereals. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2008. 25:997–1006.

19. Turner NW, Subrahmanyam S, Piletsky SA. Analytical methods for determination of mycotoxins: a review. Anal Chim Acta. 2009. 632:168–180.

20. Visconti A, Pascale M. Determination of zearalenone in corn by means of immunoaffinity clean-up and high-performance liquid chromatography with fluorescence detection. J Chromatogr A. 1998. 815:133–140.

21. Yang H, Qu L, Wimbrow AN, Jiang X, Sun Y. Rapid detection of Listeria monocytogenes by nanoparticle-based immunomagnetic separation and real-time PCR. Int J Food Microbiol. 2007. 118:132–138.

22. Yantasee W, Warner CL, Sangvanich T, Addleman RS, Carter TG, Wiacek RJ, Fryxell GE, Timchalk C, Warner MG. Removal of heavy metals from aqueous systems with thiol functionalized superparamagnetic nanoparticles. Environ Sci Technol. 2007. 41:5114–5119.

23. Yin YN, Yan LY, Jiang JH, Ma ZH. Biological control of aflatoxin contamination of crops. J Zhejiang Univ Sci B. 2008. 9:787–792.

24. Zmudzki J, Wiśniewska-Dmytrow H. Limits and regulations for mycotoxins in food and feed. Pol J Vet Sci. 2004. 7:211–216.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download