Abstract
Although ionizing radiation is known to induce cellular senescence in vitro and in vivo, its long-term in vivo effects are not well defined. In this study, we examined the prolonged expression of senescence markers in mice irradiated with single or fractionated doses. C57BL/6 female mice were exposed to 5 Gy of γ-rays in single or 5, 10, 25 fractions. At 2, 4, and 6 months after irradiation, senescence markers including mitochondrial DNA (mtDNA) common deletion, p21, and senescence-associated β-galactosidase (SA β-gal) were monitored in the lung, liver, and kidney. Increases of mtDNA deletion were detected in the lung, liver, and kidney of irradiated groups. p21 expression and SA β-gal staining were also increased in the irradiated groups compared to the non-irradiated control group. Increases of senescence markers persisted up to 6 months after irradiation. Additionally, the extent of mtDNA deletion and the numbers of SA β-gal positive cells were greater as the number of radiation fractions increased. In conclusion, our results showed that ionizing radiation, especially that delivered in fractions, can cause the persistent upregulation of senescence marker expression in vivo. This should be considered when dealing with chronic normal tissue injuries caused by radiation therapy or radiation accidents.
Aging or senescence occurs at the organ and cellular levels. Most senescent cells are characterized by morphological changes resulting in large, flat, and multinucleated phenotypes [6]. In addition, senescence cells show a stable and long-term loss of proliferative capacity although cell viability and metabolic activity are maintained. In vivo senescence has been studied using various animal models such as Drosophila melanogaster and Caenorhabditis elegans due to their short lifespan and fast breeding times [1,4]. Rodents, especially mice, have also been used given their high genetic, physiological, and anatomical similarities to humans. Long-living mutant mice such as Snell dwarf, Ames dwarf, IGF-1R hemizygous, and Klotho transgenic mice as well as senescence-accelerated mice including SOD1 knockout, PrxII knockout, and SAM-P8 mice have provided good animal models for elucidating the aging mechanism and developing anti-aging interventions [8,9,19,22,33]. However, the mechanisms of senescence in vivo remain controversial although it appears that aging is associated with several factors including telomere shortening [11], mitochondria dysfunction [30], and accumulation of DNA damage [34].
Ionizing radiation (IR) is a well-known inducer of oxidative stress and DNA damage, which are important factors for promoting the aging process. In fact, IR is known to trigger the DNA damage response leading to premature senescence along with apoptosis and growth arrest both in vitro and in vivo [10,24]. Interestingly, responses to IR in vivo are reported to be tissue-specific and dose-dependent, and the proliferative capability and structural role of the affected tissues seem to be determining factors that govern the responses to IR [10]. However, the mechanism underlying tissue- or cell-specific responses to IR remains largely unknown. In terms of the long-term health effects of IR, human studies on radiation therapy patients and Japanese atomic bomb survivors have suggested that IR is associated with aging and aging-related diseases [27]. There are also plenty of in vitro investigations and several short-term animal studies demonstrating IR can induce premature senescence [20,23,36]. However, the long-term effects of IR on tissue senescence in irradiated animals have not been studied in depth. At least one group reported the long-term expression of senescence markers like DNA damaged foci and p16 in the tissues of mice 12 weeks after exposure to a single sublethal dose of total body irradiation [16].
The present study was conducted to find out whether IR can induce or accelerate the aging process in mice by investigating the long-term effects of IR on the expression of various senescence markers in different tissues. In addition, the effect of irradiation delivery, such as single or fractionated doses, on senescence marker expression was evaluated. We previously reported that more prominent prolonged effects on immunological functions and lipid metabolism are observed when irradiation is given in fractionated doses than in a single dose [14,25]. For our investigation, mice were given single or fractionated doses of IR (in a total dose of 5 Gy), and senescence marker expression in the mouse tissues was analyzed 2, 4, and 6 months after irradiation. The well-known senescence markers we evaluated were mitochondrial DNA (mtDNA) common deletion [35], cyclin-dependent kinase inhibitor 1A (p21) expression [21], and senescence-associated β-galactosidase (SA β-gal) [5]. Our results showed that expression of these senescence markers was increased by IR and remained elevated in the mouse tissues for as long as 6 months after irradiation. Furthermore, fractionated IR delivery was more efficient than the single dose for inducing the development of these senescent phenotypes.
Female C57BL/6 (H-2b) mice 7 weeks old were purchased from Orient Bio (Korea) and used for the experiment after 1 week of quarantine and adaptation. The mice were housed five to 10 animals per cage under specific pathogen-free conditions at 22 ± 2℃ and 55~60% relative humidity. The mice were fed a standard animal diet (5L79; Orient Bio, Korea) and water ad libitum. All animal experiments were performed according to the guidelines for the use and care of laboratory animals of Ministry of Health and Welfare (Korea), and were approved by the Animal Care and Use Committee of the Korea Atomic Energy Research Institute (KAERI).
The mice were irradiated with 137Cs γ-rays using a Gammacell 40 Exactor (Nordion, Canada) at the KAERI. The protocol used for mouse irradiation and analysis is summarized in Fig. 1. Groups of 8-week-old C57BL/6 mice (nine mice per group) were exposed to whole-body irradiation delivered in a single dose (5 Gy) or fractionated doses (1 Gy × five times, 0.5 Gy × 10 times, or 0.2 Gy × 25 times). The dose rate for delivering 5 Gy or 1 Gy irradiation was 1 Gy/min. To minimize experimental error, the dose rate was reduced to 0.1 Gy/min using an attenuator when 0.5 Gy or 0.2 Gy was delivered. At 2, 4, and 6 months after irradiation, the mice were sacrificed and lung, liver, and kidney tissues were isolated. Non-irradiated two-month-old mice and 24-month-old mice were used as young and old controls, respectively. Tissues isolated from the mice were immediately frozen in liquid nitrogen and stored at -80℃ until use.
The tissues (50~100 mg) were homogenized for 30 sec in 600 µL of digestion buffer (100 mM NaCl, 10 mM Tris-Cl, pH 8.0; 25 mM EDTA, pH 8.0; 0.5% SDS, and 0.1 mg/mL proteinase K) and incubated at 50℃ for 18 h. Total DNA was isolated by phenol-chloroform-isoamyl alcohol extraction method and dissolved in 50 µL of Tris-EDTA buffer (10 mM Tris-Cl, pH 7.4; 1 mM EDTA). The DNA from three mice in each group was pooled together and used to detect mtDNA and its common deletion with nested PCR.
The mtDNA common deletion (3867 bp) was detected as previously described [35,38]. mtDNA was identified by PCR amplification of a 355-bp constant region of mtDNA using P1 and P2 (5' CGCTCTACTCACCATCTCTT 3' and 5' TGCTTACCTTGTTACGACTTA 3') primers. To confirm the presence of the deletion, nested PCR was carried out using P3/P4 (5' AATTACAGGCTTCCGACA CA 3'/5' TTTAGGCTTAGGTTGAGAGA 3') and P5/P6 (5' ACCAACAGCTACCATTACATT 3'/5' TGATTGGG TTTATGAGGTCTG 3') primer pairs. PCR with P1/P2 or P3/P4 primer pair was performed in 30 µL reaction mixture containing 1 µg of total DNA, 1 U Taq polymerase (Neurotics, Korea), 1× Taq buffer (Neurotics, Korea), 200 µM dNTPs (Neurotics, Korea), and 10 pmol of each primer. The 0.5 µL of PCR product mixture obtained from the PCR with P3/P4 primer pair was used for the DNA template of the second nested PCR with P5/P6 primer pair. After an initial denaturation (94℃, 10 min), PCR conditions for the different primer pairs were as follows: [P1/P2] 30 cycles of denaturation at 94℃ for 30 sec, annealing at 55℃ for 20 sec, and elongation at 72℃ for 30 sec; [P3/P4] 35 cycles of denaturation at 94℃ for 1 min, annealing at 55℃ for 1 min, and elongation at 72℃ for 2 min; [P5/P6] 30 cycles of denaturation at 94℃ for 1 min, annealing at 55℃ for 1 min, and elongation at 72℃ for 1 min. All PCR programs concluded with a final elongation at 72℃ for 10 min. The PCR products were electrophoresed in a 1.2% agrarose gel and stained with ethidium bromide. The DNA bands were visualized under UV light using Kodak EDAS 290 digital imaging system (Eastman Kodak, USA). Intensity of the amplified DNA bands was quantified using Kodak ID 3.6 software (Eastman Kodak, USA).
The tissues were homogenized with Omni TH tissue homogenizer (Omni International, USA) in a 1 mL of homogenization buffer [50 mM Tris-Cl, pH 8.0; 150 mM NaCl; 0.2% SDS; 1% NP-40; 5 mM NaF; 1 mM Na3VO4; 2 mM EGTA; and proteinase inhibitor (Sigma-Aldrich, USA)]. After centrifuging the mixture at 13,000 × g at 4℃ for 20 min, the supernatant was collected and its protein concentration was determined using a bicinchoninic acid kit (Thermo Fisher Scientific, USA). Tissue lysates obtained from three mice of each group were pooled together. Next, 25 µg protein were resolved with SDS-PAGE and transferred onto polyvinylidene fluoride membranes (GE healthcare, UK). The membranes were blocked in 5% skim milk at room temperature for 2 h and incubated with an anti-p21 mouse IgG antibody (1 : 1,000 dilution; BD Biosciences, USA) overnight at 4℃. After washing with TBST (Tris-buffered saline containing 0.1% Tween 20), the membranes were incubated with an HRP-conjugated anti-mouse IgG antibody (1 : 2,500 dilution; Cell Signaling Technologies, USA) at room temperature for 1 h. The membranes were washed in TBST, and the immunoreactive bands were then detected using an Immobilon chemiluminescent detection kit (EMD Millipore, USA). Densitometry to measure intensities of the protein bands on X-ray film (Fujifilm, Japan) was performed with Kodak ID 3.6 software.
SA β-gal staining in the kidneys was performed as previously described [5] with some modifications. Briefly, kidney tissue samples were snap-frozen in an optimal cutting temperature compound (Sakura Finetek, Japan). Fresh frozen sections (7-µm thick) were cut with automatic cryocut microtome (Leica Biosystems, Germany), mounted on silane-coated glass slides (Paul Marienfeld, Germany), and fixed in 10% formalin for 10 min at room temperature. The sections were washed in phosphate buffered saline, incubated in staining solution (1 mg/mL X-gal, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 2 mM MgCl2, 150 mM NaCl, and citric acid-sodium phosphate, pH 6.0) at 37℃ for 16 h, and then counterstained with eosin (Junsei Chemical, Japan). After photographing the slides with a Leica DFC500 R2 digital camera (Leica Microsystems, Germany), all cells and ones positive for SA β-gal were counted using ImageJ software (ver. 1.38; National Institutes of Health, USA). For each mouse (n = three per group), the total number of cells analyzed was between 5,000 and 20,000.
As shown in Fig. 2A, the level of mtDNA common deletion was higher in the lung, liver, and kidney of older (24-month-old) mice compared to young (2-month-old) animals as previously reported. We next determined whether IR could induce the long-term formation of mtDNA common deletion in the mouse tissues. As shown in Fig. 2B, levels of mtDNA common deletion in the lung, liver, and kidney tended to increase in the irradiated groups compared to non-irradiated age-matched mice. Increases of mtDNA common deletion in the irradiated groups were detected up to 6 months after IR delivery. In addition, the level of mtDNA common deletion tended to be higher in the fractionated irradiation groups than in the single-dose groups. Increases over 3-fold relative to the control group were observed in only the fractionated irradiation groups.
Similar to previous reports showing that increased p21 is associated with cellular senescence, higher protein levels of p21 were observed in tissues from 24-month-old mice compared to 2-month-old animals (Fig. 3A). We next measured the expression of p21 in mice exposed to IR (Fig. 3B). In the lung, over 1.5-fold increases of p21 protein levels compared to the control group were observed in the 0.2 Gy × 25 group 2 months after IR exposure, the 5 Gy × 1 group 4 months after IR delivery, and the 5 Gy × 1, 0.5 Gy × 10, 0.2 Gy × 25 groups 6 months after irradiation. In the liver, p21 levels were increased over 2-fold in all irradiated groups 4 months after IR delivery, and by 1.5-fold in the 0.2 Gy × 25 group 6 months after IR administration. In kidney, p21 expression increased over 1.5-fold in the 0.2 Gy × 25 group 2 months after irradiation, the 1 Gy × 5 group 4 months after irradiation, and the 0.5 Gy × 10 and 0.2 Gy × 25 groups 6 months after IR delivery.
The expression of SA β-gal, a well-recognized senescence marker, was increased in the kidney of old mice compared to that of young animals as shown in Figs. 4A and C. Next, we observed increased numbers of SA β-gal-positive cells in the kidneys of mice exposed to IR (Figs. 4B and D). Two months after IR exposure, the percentage of SA β-gal-positive cells was significantly increased in all irradiated groups (0.8~7.0%) compared to the control group (0.3%). Similar effects were also observed 4 months (0.2% in the control group 0.2% vs. 0.9~3.0% for the irradiated groups) and 6 months (3.2% in the control group vs. 4.8~12.0% for the irradiated groups) after IR exposure. Interestingly, higher numbers of SA β-gal-positive cells were observed as the number of radiation fractions increased. The dependency of SA β-gal-positive cell numbers on the number of fractions was consistently observed from 2 to 6 months after IR delivery.
IR generates oxidative stress and DNA damage, and thereby causes apoptosis or premature senescence in cells and tissues [10,24]. The long-term effects of IR on the senescence or aging of cells, tissues, and organs are therefore of great importance [27]. Although many in vitro and short-term in vivo studies have shown that IR can induce premature senescence [20,23,36], long-term in vivo studies are lacking. We previously reported that IR can produce long-term alterations of immunological functions and lipid metabolism, and the fractionation scheme can modulate the effects of IR [14,25]. Therefore, the present study was performed to examine the long-term effects of IR on tissue senescence in mice up to 6 months following IR exposure. The impact of different irradiation delivery schemes (single or fractionated doses) was also evaluated.
In the current study, mtDNA common deletion, p21 expression, and SA β-gal staining were examined as in vivo senescence markers. mtDNA common deletion is the deletion of a specific sequence (4977 bp in humans and 3687 bp in mice) of the mitochondrial genome [2,26,35,38] and has been proposed as a marker of aging. mtDNA deletions are known to accumulate in human and mice tissues during the normal aging process [38] as well as stress-induced premature aging [2,26,35]. Increased expression of p21 is also a well-recognized marker of cellular senescence [12]. p21 is transcriptionally controlled by p53, and acts as a cyclin-dependent kinase inhibitor that promotes cell cycle arrest and senescence [15,21]. SA β-gal expression is widely used to identify senescent cells due to its strong correlation with senescence and relatively simple detection. However, the precise physiological association of SA β-gal with senescence is largely unclear [3,5,7,13,31].
In our study, a total IR dose of 5 Gy was chosen since this is the highest dose at which no significant additional mortality was observed for 6 months in our preliminary study (data not shown). We showed that a total of 5 Gy given in a single dose or fractionated doses could persistently induce the expression of these senescence markers in mouse tissues. Increased levels of mtDNA common deletion, p21 expression and SA β-gal staining were observed in the tissues of the irradiated groups up to 6 months after IR exposure. Although the persistent elevation of DNA damage foci and p16 expression by IR has been previously reported [16], our study is the first to demonstrate that IR also elicits the long-term induction of mtDNA common deletion, p21 expression, and appearance of SA β-gal-positive cells. These results provided additional evidence that IR can promote long-term senescence in tissues.
Temporal changes of senescence markers observed in the irradiated mice did not show clear time-dependency. The induction of mtDNA common deletion, p21 expression, and SA β-gal positivity were observed at 2 months after IR delivery and persisted up to 6 months. In contrast, a previous report showed that DNA damage foci appear shortly after IR exposure but p16 expression is increased in liver, brain, and lung tissues several weeks after IR exposure [16]. The absence of time-dependent increases of senescence markers in our study implies that the senescence markers examined in this study reached the highest expression levels within 2 months and remained relatively unchanged afterwards. Prolonged elevation of p21 and p16 levels is reported to be associated with IR-induced senescence of hematopoietic stem cells and human dental pulp stem cells [20,23,36]. Therefore, persistent elevation of p21 expression as well as mtDNA common deletion and SA β-gal staining in the liver, lung, and kidney observed in our study may be strong evidence that IR can cause long-term organ aging or senescence in mice.
Interestingly, our data also showed that fractionated irradiation doses induced the appearance of mtDNA deletion and SA β-gal staining more efficiently than a single dose. The levels of mtDNA common deletion tended to increase more in the fractionated irradiation groups than the single-dose groups given that increases over 3-fold relative to the control animals were observed in only the fractionated irradiation animals. The effect of fractionated irradiation delivery was more obvious on SA β-gal staining in the kidney. The number of SA β-gal-positive cells increased as the number of radiation fractions increased.
External factors that produce genotoxic stress like IR provoke DNA damage responses mediated by p53 in a dose- and tissue-specific manner [10]. Insufficient removal of damaged cells through apoptosis can cause the accumulation of senescent cells, leading to the development of aging-associated diseases [32]. In our study, the fractionated irradiation groups received a dose lower than 1 Gy at each delivery, which is not a sufficient dose to induce apoptosis in normal tissues [17]. Therefore, it is possible that persistent exposure to a low dose of radiation does not eliminate damaged cells by apoptosis but instead results in the accumulation of damaged cells. Thus, it would be interesting to compare the effects of single and fractionated doses of radiation on the balance of apoptotic and senescent events during IR-induced tissue aging. In fact, it was recently reported that prolonged DNA damage responses can promote senescence by inducing the expression of senescence-associated inflammatory cytokines [28,37]. A persistent inflammatory response is a candidate mechanism underlying IR-induced disorders and premature aging [29]. Epidemiologic studies on cancer patients and atomic bomb survivors showed that moderate or low doses of radiation can induce non-cancer disorders, such as cardiovascular diseases, which are associated with chronic inflammation [18,27]. Therefore, it would be interesting to study the long-term relationship between IR-induced senescence and inflammation in vivo.
In conclusion, we demonstrated that IR can promote the prolonged induction of senescence marker expression in various tissues of mice. Additionally, fractionated doses of irradiation resulted in more marked induction of mtDNA deletion accumulation and SA β-gal staining than a single dose of IR. Senescence or aging induced by IR is an important issue in terms of normal tissue damage during radiation therapy, and occupational or accidental exposure to IR [27]. Our study provided evidence of prolonged in vivo senescence induction by radiation. The senescence markers and animal model we used are also applicable for studying the long-term effects of IR on in vivo tissue and organ aging.
Figures and Tables
 | Fig. 1Schematic illustration of irradiation delivery and experimental schedule. 8-week-old female C57BL/6 mice were irradiated with a single dose (5 Gy × 1) or fractionated doses (0.2 Gy × 25, 0.5 Gy × 10, or 1 Gy × 5) of ionizing radiation (IR). The mice were then sacrificed at 2, 4, and 6 months after the first exposure to radiation. |
 | Fig. 2Induction of mitochondrial DNA (mtDNA) common deletion (3867 bp) in the tissues of irradiated C57BL/6 mice. (A) Tissues isolated from young (2-month-old) and old (24-month-old) animals were analyzed for mtDNA common deletion. (B) 8-week-old mice were exposed to IR delivered in a single dose (5 Gy × 1) or fractionated doses (0.2 Gy × 25, 0.5 Gy × 10, or 1 Gy × 5), and sacrificed 2, 4, and 6 months after IR exposure. |
 | Fig. 3Increased expression of p21 in tissues of irradiated C57BL/6 mice. (A) Tissues isolated from young (2-month-old) and old (24-month-old) mice were analyzed for p21 protein expression. (B) 8-week-old C57BL/6 mice were exposed to a single dose (5 Gy × 1) or fractionated doses (0.2 Gy × 25, 0.5 Gy × 10, or 1 Gy × 5) of IR, and sacrificed at 2, 4, 6, months after radiation exposure. |
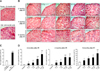 | Fig. 4Increased numbers of senescence-associated β-galactosidase (SA β-gal)-positive cells in the kidney of irradiated C57BL/6 mice. (A and C) Kidneys isolated from young (2-month-old) and old (24-month-old) C57BL/6 mice were stained for SA β-gal. (B and D) Mice were exposed to IR delivered in a single dose (5 Gy × 1) or fractionated doses (0.2 Gy × 25, 0.5 Gy × 10, or 1 Gy × 5), and sacrificed at 2, 4, and 6 months after radiation exposure. Cells stained blue are positive for SA β-gal. Significant differences relative to the control group are indicated (*p < 0.05 and **p < 0.01). Scale bars = 20 µm. |
Acknowledgments
This study was supported by the Nuclear R&D Program of Ministry of Science and Technology, Korea (Project No. 2007-2000091).
References
1. Barsyte D, Lovejoy DA, Lithgow GJ. Longevity and heavy metal resistance in daf-2 and age-1 long-lived mutants of Caenorhabditis elegans. FASEB J. 2001. 15:627–634.

2. Berneburg M, Plettenberg H, Medve-König K, Pfahlberg A, Gers-Barlag H, Gefeller O, Krutmann J. Induction of the photoaging-associated mitochondrial common deletion in vivo in normal human skin. J Invest Dermatol. 2004. 122:1277–1283.

3. Choi J, Shendrik I, Peacocke M, Peehl D, Buttyan R, Ikeguchi EF, Katz AE, Benson MC. Expression of senescence-associated beta-galactosidase in enlarged prostates from men with benign prostatic hyperplasia. Urology. 2000. 56:160–166.

4. Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001. 292:104–106.

5. Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campisi J. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995. 92:9363–9367.

7. Gresh L, Fischer E, Reimann A, Tanguy M, Garbay S, Shao X, Hiesberger T, Fiette L, Igarashi P, Yaniv M, Pontoglio M. A transcriptional network in polycystic kidney disease. EMBO J. 2004. 23:1657–1668.

8. Han YH, Kim HS, Kim JM, Kim SK, Yu DY, Moon EY. Inhibitory role of peroxiredoxin II (Prx II) on cellular senescence. FEBS Lett. 2005. 579:4897–4902.

9. Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in Ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci. 2003. 58:291–296.

10. Jackson JG, Post SM, Lozano G. Regulation of tissue- and stimulus-specific cell fate decisions by p53 in vivo. J Pathol. 2011. 223:127–136.
11. Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, Sahin E, Kost-Alimova M, Protopopov A, Cadiñanos J, Horner JW, Maratos-Flier E, Depinho RA. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011. 469:102–106.

12. Jeyapalan JC, Sedivy JM. Cellular senescence and organismal aging. Mech Ageing Dev. 2008. 129:467–474.

13. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002. 418:41–49.

14. Jo SK, Seol MA, Park HR, Jung U, Roh C. Ionising radiation triggers fat accumulation in white adipose tissue. Int J Radiat Biol. 2011. 87:302–310.

15. Kailong L, Du X, Yani H, Lin Z, Jvrong Y, Ruihua S, Lin C. p53-Rb signaling pathway is involved in tubular cell senescence in renal ischemia/reperfusion injury. Biocell. 2007. 31:213–223.

16. Le ONL, Rodier F, Fontaine F, Coppe JP, Campisi J, DeGregori J, Laverdière C, Kokta V, Haddad E, Beauséjour CM. Ionizing radiation-induced long-term expression of senescence markers in mice is independent of p53 and immune status. Aging Cell. 2010. 9:398–409.

17. Ling CC, Guo M, Chen CH, Deloherey T. Radiation-induced apoptosis: effects of cell age and dose fractionation. Cancer Res. 1995. 55:5207–5212.
18. Little MP. Cancer and non-cancer effects in Japanese atomic bomb survivors. J Radiol Prot. 2009. 29:A43–A59.

19. Maruyama N, Ishigami A, Kuramoto M, Handa S, Kubo S, Imasawa T, Seyama K, Shimosawa T, Kasahara Y. Senescence marker protein-30 knockout mouse as an aging model. Ann N Y Acad Sci. 2004. 1019:383–387.

20. Meng A, Wang Y, Van Zant G, Zhou D. Ionizing radiation and busulfan induce premature senescence in murine bone marrow hematopoietic cells. Cancer Res. 2003. 63:5414–5419.
21. Mirzayans R, Andrais B, Scott A, Paterson MC, Murray D. Single-cell analysis of p16INK4a and p21WAF1 expression suggests distinct mechanisms of senescence in normal human and Li-Fraumeni syndrome fibroblasts. J Cell Physiol. 2010. 223:57–67.
23. Muthna D, Soukup T, Vavrova J, Mokry J, Cmielova J, Visek B, Jiroutova A, Havelek R, Suchanek J, Filip S, English D, Rezacova M. Irradiation of adult human dental pulp stem cells provokes activation of p53, cell cycle arrest, and senescence but not apoptosis. Stem Cells Dev. 2010. 19:1855–1862.

24. Oh CW, Bump EA, Kim JS, Janigro D, Mayberg MR. Induction of a senescence-like phenotype in bovine aortic endothelial cells by ionizing radiation. Radiat Res. 2001. 156:232–240.

25. Park HR, Jo SK, Eom HS. Chronic effects of single and fractionated γ-irradiation on an impairment of Th1-related immune response. Int J Radiat Biol. 2011. 87:534–543.

26. Prithivirajsingh S, Story MD, Bergh SA, Geara FB, Ang KK, Ismail SM, Stevens CW, Buchholz TA, Brock WA. Accumulation of the common mitochondrial DNA deletion induced by ionizing radiation. FEBS Lett. 2004. 571:227–232.

27. Richardson RB. Ionizing radiation and aging: rejuvenating an old idea. Aging (Albany NY). 2009. 1:887–902.

28. Rodier F, Coppé JP, Patil CK, Hoeijmakers WAM, Muñoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009. 11:973–979.

29. Sabin RJ, Anderson RM. Cellular senescence - its role in cancer and the response to ionizing radiation. Genome Integr. 2011. 2:7.

30. Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010. 464:520–528.

31. Severino J, Allen RG, Balin S, Balin A, Cristofalo VJ. Is β-galactosidase staining a marker of senescence in vitro and in vivo? Exp Cell Res. 2000. 257:162–171.
33. Vergara M, Smith-Wheelock M, Harper JM, Sigler R, Miller RA. Hormone-treated Snell dwarf mice regain fertility but remain long lived and disease resistant. J Gerontol A Biol Sci Med Sci. 2004. 59:1244–1250.

34. Wang C, Jurk D, Maddick M, Nelson G, Martin-Ruiz C, von Zglinicki T. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell. 2009. 8:311–323.

35. Wang L, Kuwahara Y, Li L, Baba T, Shin RW, Ohkubo Y, Ono K, Fukumoto M. Analysis of common deletion (CD) and a novel deletion of mitochondrial DNA induced by ionizing radiation. Int J Radiat Biol. 2007. 83:433–442.

36. Wang Y, Schulte BA, LaRue AC, Ogawa M, Zhou D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood. 2006. 107:358–366.

37. Zhan H, Suzuki T, Aizawa K, Miyagawa K, Nagai R. Ataxia telangiectasia mutated (ATM)-mediated DNA damage response in oxidative stress-induced vascular endothelial cell senescence. J Biol Chem. 2010. 285:29662–29670.

38. Zhang X, Han D, Ding D, Dai P, Yang W, Jiang S, Salvi RJ. Cochlear mitochondrial DNA3867bp deletion in aged mice. Chin Med J (Engl). 2002. 115:1390–1393.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download