Abstract
Here, we describe two dogs in which canine small intestinal submucosa (SIS) was implanted as a biomaterial scaffold during perineal herniorrhaphy. Both dogs had developed severe muscle weakness, unilaterally herniated rectal protrusions, and heart problems with potential anesthetic risks. Areas affected by the perineal hernia (PH) located between the internal obturator and external anal sphincter muscles were reconstructed with naïve canine SIS sheets. In 12 months, post-operative complications such as wound infections, sciatic paralysis, rectal prolapse, or recurrence of the hernia were not observed. Symptoms of defecatory tenesmus also improved. Neither case showed any signs of rejection or specific immune responses as determined by complete and differential cell counts. Our findings demonstrate that canine SIS can be used as a biomaterial scaffold for PH repair in dogs.
Perineal hernia (PH) results from a failure of the pelvic muscle diaphragm structures to support the pelvic and caudal abdominal contents (rectum, bladder, and small intestine). Consequently, these contents extend and deviate into the subcutaneous perineal region. The prevalence of PH in dogs of hospital accessions is relatively low (0.1%~0.4%), especially among females. However, this condition commonly develops in middle-aged and elderly male dogs [4]. Muscle deterioration caused by PH mainly affects the area between the external anal sphincter, the levator ani, and coccygeal muscles [7]. This progressive shrinkage of muscle fibers is possibly associated with imbalances of gonadal hormones, such as estrogen, androgen, and relaxin, related to prostatic hypertrophy [13,17].
Standard herniorrhaphy (a simple appositional technique) has been associated with postoperative complication rates ranging from 28.6% to 61%, and a recurrence rate of 10% [3,5]. Alternative techniques have been developed to improve hernial repair procedures and decrease the recurrence rate. Internal obturator muscle transposition (IOT) is currently the preferred surgical treatment (with recurrence rates between 2.4% and 19%), but repair can be difficult, particularly if the internal obturator muscle is atrophied [9,18]. Post-operative complications with the use of standard herniorrhaphy including IOT, and peripheral muscle autografts (e.g., semitendinous muscle flap or superficial gluteal muscle transposition) have been reported. These include sciatic nerve injury, fecal incontinence, infection around the incision site, prolapsed rectum with excessive straining, misplacement of sutures in the anal sac or rectal lumen, urinary bladder necrosis, urinary incontinence, and PH recurrence [1,12].
Ideally, herniorrhaphy should be easy to perform, and repair the defect with sufficient strength to avoid recurrence and minimize complications. For this, some researchers have recommended the use of prosthetic implants [synthetic polypropylene (PP) mesh] or bioscaffolds such as dermal collagen sheets [8] and small intestinal submucosa (SIS) derived from pigs [19]. In the current study, we prepared naïve canine SIS bioscaffolds from a donated German shepherd cadaver within 2 h of euthanasia following a previously described method [2]. This naïve graft was applied to the PH site in two dogs undergoing a perineal herniorrhaphy.
Case 1: A 12-year-old, intact male Shih Tzu weighing 4.4 kg was referred to the Veterinary Teaching Hospital of Konkuk University (Korea) with a 6-month history of rectal dyschezia, vomiting, and anorexia associated with a PH.
On presentation, the dog was alert but had a grade (III) heart murmur. Swelling lateral to and extending some distance from the right craniodorsal area to the anus was evident. The area of hernial swelling (5 × 5 × 3 cm) was hard, but the site was soft and could be pushed back into the pelvic after stool was removed from the rectum. A lateral deviation of the rectum was detected in the herniated area by rectal examination. Ultrasonography and radiography revealed a protrusion of the rectum and fatty tissue (Fig. 1A). Differential diagnoses included rectal sacculation, rectal diverticulum, and neoplastic rectal diseases. Therefore, magnetic resonance imaging (MRI) was performed with a 3T scanner (Medinus, Korea). T1- and T2-weighted images as well as T1-enhanced images were obtained. Contrast agent (0.2 mL/kg, Magnevist; Bayer Schering Pharma, Germany) was intravenously injected for the T1-enhanced imaging. MRI revealed a weakness in the perineal muscles (internal obturator, levator ani, coccygeal, and obturator) and an abnormally enlarged rectal dilation between the muscles. Canine prostatic hyperplasia was confirmed under the dilated rectum (Fig. 1B).
The dog received a pre-operative intramuscular (IM) injection of 10 µg/kg buprenorphine (Renolphan; Hanlim Pharm, Korea) and an intravenous (IV) injection of 0.3 mg/kg midazolam (Vascam; HaNa Pharm, Korea). General anesthesia was induced with an IV injection of 6 mg/kg propofol (Anepol; HaNa Pharm, Korea). Inhalation anesthesia was maintained using a combination of 2% isoflurane (Forane; ChoongWae Pharma, Korea) and 100% oxygen. Epidural anesthesia was induced using a mixture of 0.5 mg/kg bupivaccaine (Bupivacaine 0.5%; HaNa Pharm, Korea) and 10 µg/kg buprenorphine (Renolphan; Hanlim Pharm, Korea). A half-circle skin incision was made over the hernia. The muscles were atrophied and intermingled with omentum-like fatty tissue. The fatty tissue was ligated and removed as necessary. The herniated organs were replaced into the pelvic cavity by gentle manipulation, and a clear view of the funnel-shaped tunnel between the perineal muscles was then obtained (Fig. 2A).
Naïve canine SIS consisting of four-layered sheets (40 × 20 mm) was inserted between the levator ani, coccygeus, and internal obturator muscles, and secured with monofilament polyglyconate sutures (4-0 Maxon; Covidien, USA) placed in a simple interrupted pattern (Fig. 2B). Another series of sutures (4-0 Maxon; Covidien, USA) was placed in the subcutaneous tissues, and skin was closed with monofilament polyamide sutures (3-0 Dafilon; B. Braun Melsungen, Germany). Castration was performed because of the muscle dilation and weakness-related canine prostatic hyperplasia.
Buprenorphine (10 µg/kg, IM) as an analgesia was administered postoperatively every 8 h for 2 days and treatment with cephalexin (22 mg/kg) was continued twice per day for 14 days. After surgery, a complete blood count (CBC), differential count (DC), and serum biochemistry profile were obtained daily for 14 days; all results were within normal ranges. A mild rectal protrusion from the left side opposite to the surgical site was developed and identified 14 days post-operatively. This was probably due to severe rectal dilation with long-term fecal accumulation before surgery. We also suspected that the left side muscles to the anus in PH area were previously severely weakened by prostatic enlargement and hormone imbalances like relaxin [13,17]. We were concerned that the canine could have experienced anesthesia-related problems such as heart abnormalities and high blood pressure. Therefore, we only prescribed a stool softener to prevent PH recurrence, and the patient did not show deviation of the rectum to the left side. Twelve months after herniorrhaphy, the dog remained clinically normal and no complications were reported by the owner.
Case 2: An 11-year-old, castrated male Yorkshire terrier weighing 3.38 kg was referred to the Veterinary Teaching Hospital of Konkuk University (Korea) with a right-sided PH (4.9 × 4.9 × 4.8 cm), bladder/urethral calculi causing defecation/urination difficulties, and clinical signs of a heart problem (mitral valve insufficiency). Irregular surfaces of the urethral calculi were immediately proximal to the os penis and cystic calculi were also observed. In addition, rectal deviation was identified on the right perineal area by radiography (Fig. 3A). After hydropropulsion, all urethral calculi returned to the bladder. Because the patient had a heart problem and was scheduled to undergo cystotomy with herniorrhaphy, our team should have reduced the time taken to operate.
Prior to surgery, the patient received buprenorphine (10 µg/kg, IM) and midazolam (0.3 mg/kg, IV). General anesthesia was induced with IV injection of 2 mg/kg etomidate (Etomidate-lipuro; B. Braun Melsungen, Germany). Inhalation anesthesia was maintained using a combination of 2% isoflurane and 100% oxygen. Epidural anesthesia was induced before herniorrhaphy. The urethral cystic caculi were removed by cystotomy and found to be composed of calcium oxalate. At the PH surgical site, the herniated rectum and fatty tissue organs were replaced into the pelvic cavity. Canine SIS sheets (40 × 30 mm) were inserted between the perineal muscles, and secured with 4-0 Maxon sutures (Covidien, USA) placed in a simple interrupted pattern similar to Case 1. PH repair with canine SIS was completed in about 40 min (Fig. 3B). No difficulties in defecating or urinating were observed after the operation. CBC and DC values were within normal ranges 14 days after surgery. The surgical site has been well preserved for the past year.
Four types of PH have been previously described: caudal, dorsal, ventral, and sciatic. The variety of techniques used to repair PH in dogs reflects the difficulty of this type of surgical procedure [15]. Scaffolds used for perineal reconstruction and replacement should have structural and functional properties appropriate for treating all types of PH. Moreover, there is no need to require additional dissection, these scaffolds replacement could be faster than those with the IOT technique.
PP is one of the most commonly used synthetic materials for herniorrhaphy, and its application has been investigated in numerous experimental settings. However, complications reported such as local wound disturbances, seromas, adhesions, and stiffness in humans [16]. Adhesion formation is major problem associated with synthetic mesh and this type of inflammatory reaction is due to increased amounts of connective tissue, which does not necessarily translate into greater strength and durability of the hernia repair [14]. Therefore, studies of alternative biologic scaffold materials have been conducted in animals and clinical settings.
A previous preclinical study has shown that there were no significant differences among the four-layered porcine SIS herniorrhaphy group, the IOT techniques group, and the non-operated normal dog group. On the other hand, SIS herniorrhaphy is easy to perform and is associated with fewer potential complications such as inflammation, mineralization, and necrosis [19]. Porcine SIS is a resorbable xenogeneic bioscaffold that induces tissue and constructive remodeling in animal models of vascular grafts [2], urinary bladder repair [11], and treatment of body wall defects [6]. SIS consists primarily of an extracellular matrix composed of collagen type I, glycosaminoglycans, proteoglycans, and glycoproteins [10]. This scaffold also contains factors associated with cell signaling (epidermal growth factor, fibroblast growth factor-2, and vascular endothelial growth factor), which are involved in angiogenesis, cell migration, and differentiation [20].
We implanted canine SIS in two dogs and expected that this would provide improved outcomes because SIS should result in a stronger repair than muscular repair techniques. Furthermore, canine SIS was an allograft and therefore associated with fewer complications related to immunologic responses.
Our cases showed that the canine SIS sheets were easily secured to the PH sites, resulting in a strong repair. Additionally, no PH recurrence or other complications, including wound infection, rectal prolapsed, or following breakdown of the repair, urinary tract malfunction, neurapraxia, or tenesmus, were observed within 12 months after surgery. Our results demonstrated that this biomaterial is an appropriate alternative for synthetic meshes and the IOT technique. Further clinical evaluation will lead to an improved understanding of how bioscaffold materials promote the healing process.
Figures and Tables
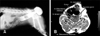 | Fig. 1In Case 1, Positive contrast radiograph and magnetic resonance imaging scans. (A) Lateral view showed that the rectum protruded into the hernia sac by barium enema. (B) The levator ani, coccygeal, and obturator muscles appeared around the dilated rectum, and the canine prostate was found to be hyperplastic on the T1-weighted image. |
 | Fig. 2Intraoperative photographs of perineal herniorrhaphy using canine small intestinal submucosa (SIS) in Case 1. (A) The four-layered canine SIS sheet was prepared. After placing the deviated rectum in its original location, the levator ani, coccygeus, and internal obturator muscles were identified. (B) The canine SIS sheet was placed between the perineal muscles. |
Acknowledgments
The authors would like to thank the surgeons at the Veterinary Teaching Hospital of Konkuk University, Korea for their help with managing these cases.
References
1. Anderson MA, Constantinescu GM, Mann FA. Bojrab MJ, Ellison GW, Slocum B, editors. Perineal hernia repair in the dog. Current Techniques in Small Animal Surgery. 1998. 4th ed. Philadelphia: Lippincott Williams & Wilkins;555–564.
2. Badylak SF, Lantz GC, Coffey A, Geddes LA. Small intestinal submucosa as a large diameter vascular graft in the dog. J Surg Res. 1989. 47:74–80.

4. Bellenger CR, Canfield RB. Slatter DH, editor. Perineal hernia. Textbook of Small Animal Surgery. 2003. 3rd ed. Philadelphia: Saunders;487–498.
6. Clarke KM, Lantz GC, Salisbury SK, Badylak SF, Hiles MC, Voytik SL. Intestine submucosa and polypropylene mesh for abdominal wall repair in dogs. J Surg Res. 1996. 60:107–114.

7. Dorn AS, Cartee RE, Richardson DC. A preliminary comparison of perineal hernia in the dog and man. J Am Anim Hosp Assoc. 1982. 18:624–632.
8. Frankland AL. Use of porcine dermal collagen in the repair of perineal hernia in dogs-a preliminary report. Vet Rec. 1986. 119:13–14.

9. Hardie EM, Kolata RJ, Earley TD, Rawlings CA, Gorgacz EJ. Evaluation of internal obturator muscle transposition in treatment of perineal hernia in dogs. Vet Surg. 1983. 12:69–72.

10. Hodde JP, Badylak SF, Brightman AO, Voytik-Harbin SL. Glycosaminoglycan content of small intestinal submucosa: a bioscaffold for tissue replacement. Tissue Eng. 1996. 2:209–217.

11. Kropp BP, Eppley BL, Prevel CD, Rippy MK, Harruff RC, Badylak SF, Adams MC, Rink RC, Keating MA. Experimental assessment of small intestinal submucosa as a bladder wall substitute. Urology. 1995. 46:396–400.

12. Matthiesen DT. Diagnosis and management of complications occurring after perineal herniorrhaphy in dogs. Compend Contin Educ Pract Vet. 1989. 11:797–802.
13. Niebauer GW, Shibly S, Seltenhammer M, Pirker A, Brandt S. Relaxin of prostatic origin might be linked to perineal hernia formation in dogs. Ann N Y Acad Sci. 2005. 1041:415–422.

14. Novitsky YW, Harrell AG, Cristiano JA, Paton BL, Norton HJ, Peindl RD, Kercher KW, Heniford BT. Comparative evaluation of adhesion formation, strength of ingrowth, and textile properties of prosthetic meshes after long-term intra-abdominal implantation in a rabbit. J Surg Res. 2007. 140:6–11.

15. Raffan PJ. A new surgical technique for repair of perineal hernias in the dog. J Small Anim Pract. 1993. 34:13–19.

17. Shahar R, Shamir MH, Niebauer GW, Johnston DE. A possible association between acquired nontraumatic inguinal and perineal hernia in adult male dogs. Can Vet J. 1996. 37:614–616.
18. Sjollema BE, van Sluijs FJ. Perineal hernia repair in the dog by transposition of the internal obturator muscle. II. Complications and results in 100 patients. Vet Q. 1989. 11:18–23.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download