Abstract
The purpose of this study was to evaluate the capacity of a lactic acid bacteria (LAB) inoculum to protect calves with or without lactose supplements against Salmonella Dublin infection by evaluating histopathological lesions and pathogen translocation. Fifteen calves were divided into three groups [control group (C-G), a group inoculated with LAB (LAB-G), and a group inoculated with LAB and given lactose supplements (L-LAB-G)] with five, six, and four animals, respectively. The inoculum, composed of Lactobacillus (L.) casei DSPV 318T, L. salivarius DSPV 315T, and Pediococcus acidilactici DSPV 006T, was administered with milk replacer. The LAB-G and L-LAB-G received a daily dose of 109 CFU/kg body weight of each strain throughout the experiment. Lactose was provided to the L-LAB-G in doses of 100 g/day. Salmonella Dublin (2 × 1010 CFU) was orally administered to all animals on day 11 of the experiment. The microscopic lesion index values in target organs were 83%, 70%, and 64.3% (p < 0.05) for the C-G, LAB-G, and L-LAB-G, respectively. Administration of the probiotic inoculum was not fully effective against infection caused by Salmonella. Although probiotic treatment was unable to delay the arrival of pathogen to target organs, it was evident that the inoculum altered the response of animals against pathogen infection.
Probiotics are live microorganisms that provide beneficial effects to the hosts when administered in adequate quantities [5]. The use of autochthonous microorganisms with probiotic activities provides an efficient alternative for treating and preventing some animal diseases [19]. Under normal conditions, probiotics administration would not be necessary because animals acquire the protective intestinal microorganisms directly from maternal and environmental sources. Nevertheless, intensive rearing conditions oblige farmers to wean calves early (thus limiting the contact between calves and their mothers), feed them non-natural food (e.g. replacers), and introduce them to highly stressful environments. All of these conditions make the animals more susceptible to colonization by pathogenic microorganisms.
Salmonella spp. and Escherichia coli are the most common bacterial etiologic agents of calf diarrhea during the first weeks of life [18]. Increased isolation frequency of Salmonella spp. indicates that the modern cattle breeding is favorable for development of this pathogen, especially when there are deficiencies in hygienic practices during rearing. The use of probiotic bacteria as a supplement in farm animal feeds, especially in intensive cattle production systems, is based on properties of the bacteria that improve feed nutrient conversion, and as the ability of these microorganisms to act against pathogenic bacteria [8]. At the same time, probiotic microorganisms contribute to the safety of raw materials to be used in food consumed by humans. In Argentina, there are some commercial products intended for animal feed that are marketed as beneficial supplements due to their probiotic properties. However, no probiotic inoculum isolated from the indigenous microbiota of animals belonging to national livestock farms is found in the market or has been described in the literature.
The ability of probiotic microorganisms to inhibit or counteract the negative effects of pathogens in live animals is a property that has been widely studied in laboratory animals [8,14,16] but not farm animals. Experimental models of intestinal disease could be used to evaluate the ability of an experimental probiotic inoculum to prevent the translocation of a pathogen to an internal organs and the production of lesions in calves infected with Salmonella. The animals supplemented with probiotics and lactose could have advantages in their response against intestinal pathogen.
We previously observed that some lactic acid bacteria (LAB) are capable of colonizing the intestinal tract of mice without affecting feed intake, and protecting the animals against Salmonella Dublin DSPV 595T [8,9]. In addition, LAB can colonize the gastrointestinal tract of calves without translocating to other internal organs [7] and improve the growth of dairy calves exposed to nutritional stress such as diets with high lactose contents [10,11]. The purpose of the present study was to evaluate the ability of a microbial inoculum composed of three LAB strains of bovine origin to protect young calves, artificially reared and supplemented with lactose, against Salmonella Dublin infection. This was accomplished by evaluating the development of histopathological lesions and pathogen translocation.
This study was carried out in an area designed for artificial calf rearing at the Facultad de Ciencias Veterinarias, Universidad Nacional del Litoral (Argentina), and involved 15 male calves (Bos taurus) with an average age of 5 days. All calves originated from a single dairy farm and were transported to the study site by truck (85 km). All calves received water ad libitum and were fed only milk replacer and a commercial concentrate pellet 6 h later after arrival. The calves were kept separated from each other in single cages to avoid reinfection. The breeding of animals was carried out on a dirt floor covered with natural grasses. Every week, each animal was moved to a new space with floors composed of the same type of soil and free of droppings. Throughout the experiment, all the animals were fed with milk replacer and given drinking water. The milk replacer was reconstituted at a concentration of 11% of dry matter (DM), and administered to the calves at 6:00 a.m. (2 L/animal) and 6:00 p.m. (2 L/animal) at approximately 38℃. A commercial concentrate pellet was offered to calves ad libitum throughout the experiment. This breeding procedure performed in these conditions is known as intensive rearing. The experiment lasted for 15 days and was conducted according to guidelines for the use and care of agricultural animals in agricultural research and teaching [4]. Before commencing the study, the protocol used was approved by the Advisory Committee on Ethics and Security of the Facultad de Ciencias Veterinarias, Universidad Nacional del Litoral (Argentina).
Three bacterial strains of bovine origin [Lactobacillus (L.) casei DSPV 318T, L. salivarius DSPV 315T, and Pediococcus acidilactici DSPV 006T] shown to have probiotic properties [7-11] were used for inoculation. The strains were isolated from healthy dairy calves artificially reared by a work team from the Departamento de Salud Pública Veterinaria, Facultad de Ciencias Veterinarias (Argentina), and then stored at -80℃ in de Man, Rogosa and Sharpe (MRS) medium (Britania, Argentina) containing glycerol (35% v/v). The different strains were identified using molecular biology techniques (amplification, sequencing and comparison of 16S rDNA genes) as previously described [20]. The GenBank accession numbers for the 16S rDNA genes of the three strains were FJ787305, FJ787306, and FJ787307.
The Salmonella Dublin DSPV 595T strain of bovine origin was isolated from the liver of a necropsied calf (Animal Health Hospital of the Facultad de Ciencias Veterinarias, Argentina) and stored at -80℃ in brain heart infusion (BHI) broth (Britania, Argentina) with glycerol (35% v/v). The biochemical profile of the strain was determined with an API 20 E system (bioMerieux, USA) and the phenotype was identified by the Servicio de Enterobacterias del Instituto Nacional de Enfermedades Infecciosas, Administración Nacional de Laboratorios e Instituto de Salud "Dr. Carlos G. Malbrán" (Argentina). The Genbank accession number for the 16S rDNA gene of the strain was found to be FJ997268.
Salmonella Dublin DSPV 595T was made resistant to the antibiotics novobiocin and nalidixic acid in order to be able to monitor the pathogen during the in vivo study. Resistance of the pathogen strain to the antibiotics was obtained by serial culturing [3] in xylose lysine deoxycholate (XLD) medium (Oxoid, UK) with low levels of the antibiotics up to 50 µg/mL novobiocin (Fluka, Germany) and 10 µg/mL nalidixic acid (Sigma, USA). The antibiotics were dissolved in water and prepared as 10 mg/mL stock solutions. An overnight microorganism culture was spread over XLD agar plates (Oxoid, UK) supplemented with 50 µg/mL novobiocin and 10 µg/mL nalidixic acid (XLDnov nal), and incubated for 24 h at 37℃. Isolated colonies resistant to novobiocin and nalidixic acid were cultured in BHI broth (Britania, Argentina) for 24 h at 37℃. Physiological, biochemical, and genotypic characteristics of both the original and resistant strains were compared in order to guarantee that resistance was the only observable difference. This verification was performed with biochemical tests and PCR amplification of the InvA gene specific for Salmonella spp. The Salmonella Dublin DSPV 595T strain resistant to novobiocin and nalidixic acid was stored at -80℃ (BHI broth containing glycerol 35% v/v), and later used to administered to the calves.
The calves were randomly divided into three experimental groups: the control group (C-G; five animals), the group inoculated with LAB (LAB-G; six animals), and the group inoculated with LAB and given lactose (L-LAB-G; four animals). The probiotic inoculum was administered once daily to each calf in the LAB-G and L-LAB-G groups along with milk replacer supplied in the afternoon for all 15 days of the experiment. Calves in the C-G group were inoculated in the same manner but the milk replacer was supplemented with 40 mL of 0.15 M NaCl as a placebo. Lactose (100 g/day) was administered to each L-LAB-G calf. The presence/absence of Salmonella was evaluated both before (day 0 of the experiment) and after experimental infection (day 11 of the experiment). Necropsies of one calf from each experimental group were performed daily starting from day 11 of the experiment until all calves had been sacrificed.
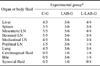
Different organs and body fluids were obtained for microbiological diagnosis. Spleen weight was measured along with body weight (BW), and the spleen weight index (SWI) was then calculated. To evaluate histopathological changes, specific indicators of lesions were identified for each organ. As a measurement of lesion severity, we used the microscopic lesion index (MLI; Table 1). Values of 1, 2, 3, and 4 were assigned according to the degree of severity (1: no apparent lesions, 2: mild lesions, 3: moderate lesions, and 4: severe lesion). This helped to standardize the routine observations and evaluate the degree of structure modification in the organs. Based on the observed indicators, the MLI values were calculated with the following formula:
MLI = [(oE1 × 1) + (oE2 × 2) + (oE3 × 3) + (oE4 × 4)] / (To × 4) × 100
in which oE1, oE2, oE3, and oE4 represent the number of organs with scores of 1, 2, 3, or 4, respectively, and To is the total number of organs analyzed in each calf (To = 7). The index values were greater as the lesion severity increased.
Bacteria were grown in MRS medium (Britania, Argentina) for 18~20 h at 37℃. The optical density of the cultures was determined at 560 nm (Metrolab 330 Spectrophotometer UV Vis; Metrolab, Argentina) and the bacterial concentration was calculated using a calibration curve as previously described [9]. The cultures were centrifuged at 3,000 × g for 10 min at 18℃, and suspended in a NaCl solution (0.15 M). Afterwards, the three strains were mixed and brought to the final volume (40 mL NaCl solution 0.15 M). The probiotic inoculum consisted of a 40-mL volume of the three microorganisms in a 0.15 M NaCl solution. The inoculum dose was 109 CFU/kg BW of each strain and administered daily via the milk replacer to calves in the LAB-G and L-LAB-G groups throughout the experiment. Control calves were inoculated with 40 mL 0.15 M NaCl solution as a placebo in the same manner.
Salmonella Dublin DSPV 595T grown in BHI broth for 18 h at 37℃ was administered via the milk replacer to all calves in the three experimental groups (C-G, LAB-G, and L-LAB-G) on day 11 of the experiment. To measure the concentration of the bacteria, a series of 10-fold dilutions were made from a Salmonella Dublin culture. Absorbance of the original culture and dilutions was measured at 560 nm (Metrolab 330 Spectrophotometer UV Vis; Metrolab, Argentina) and the number of colonies grown (CFU) on XLD plates was simultaneously determined. Regression analysis was performed with absorbance and CFU parameters. To quantify the amount of pathogen provided we used the equation:
y = 0.4735 ln(x) + 8.2162
in which y corresponds to log10 CFU/mL and x represents the absorbance of the culture. The inoculum was administered at a dose of 2 × 1010 CFU/calf. This infective dose was chosen based on publications in the literature [2,15,16] and experimental verification carried out in a previous study in two calves.
Feeds used in this study were not supplemented with antibiotics. The milk replacer contained 23% crude protein, 15% fat, 1% crude fiber, 1% calcium and 0.8% phosphorus (AF-80; ACA, Argentina). The lactose supplemented in the L-LAB-G treatment contained 99.5% lactose (Milkaut, Argentina). The commercial concentrate pellet (Cooperación; ACA, Argentina) included the following ingredients: ground corn grain, soybean pellet, wheat bran, dicalcium phosphate, sodium chloride, and a vitamin-mineral supplement. The starter contained 90% dry matter (DM), 18% crude protein, 2.9 Mcal metabolizable energy/kg DM, 80% total digestible nutrients, 5% crude fiber, 1.2% calcium, 0.8% phosphorus, and 5% ether extract. All feed composition data were obtained from the label provided by feed supplier.
Starting on day 11 of the experiment, necropsies were performed every day on one animal from each experimental group. The necropsies were performed at 22 h after the last administration of the probiotic inoculum. The animals were desensitized with a euthanasic drug (Euthanyle, 80 mg/kg; Brouwer, Argentina) administered under aseptic conditions. The animals were bled and then necropsied using conventional techniques. Samples of liver, spleen, mesenteric lymph nodes, ileocecal lymph nodes, small (jejunum and ileum) and large (cecum and colon) intestines, mediastinal lymph node, lung, and popliteal lymph nodes were collected using sterile instruments to minimize the possibility of bacterial cross-contamination between samples [13]. Samples of cerebrospinal fluid, synovial fluid, and bile were taken with sterile syringes (Jiangsu Nppo, China) with hypodermic needles (Nipro, Japan). Additionally, the ileocecal valve (IV) was removed for histopathological analysis. Spleen weight was determined along with BW, and used to calculate the SWI as follows [7]:
SWI = Spleen weight (g) / BW (kg)
Fecal samples (approximately 5 g each) were collected on days 1 and 10 of the experiment from the rectum of all calves by rectal massage. Feces were cultured either in selenite cystine broth (Britania, Argentina) for 12 h at 42℃ or Rappaport Vassiliadis broth (Merck, Germany) for 18 h at 42℃ (1 g and 0.1 g of fecal samples, respectively). After incubation, XLD agar plates were cultured and incubated for 24 h at 37℃.
Samples of liver, spleen, lung, and complete mesenteric, ileocecal, mediastinal, and popliteal lymph nodes were obtained under aseptic conditions and homogenized with a Stomacher 80 Biomaster (Seward, UK) in buffered peptone water (Britania, Argentina). After incubating for 18 h at 37℃, 1-mL aliquots of the tissue suspensions were used to inoculate 10 mL of selenite cystine broth and incubated at 42℃ for 12 h. Similarly, 0.1-mL aliquots of the suspensions were used to inoculate 10 mL of Rappaport-Vassiliadis broth and incubated at 42℃ for 18 h. Aliquots (enough to fill a bacteriological loop) of the resulting broth cultures were streaked onto XLDnov nal agar and incubated at 37℃ for 24 h.
Typical colonies with positive agglutination with a polyclonal antibody (provided by the Administración Nacional de Laboratorios e Instituto de Salud, Dr. Carlos G. Malbrán) was considered positive for Salmonella Dublin. Samples of cerebrospinal fluid, synovial fluid, and bile (1 mL each) were used to directly inoculate selenite cystine broth and Rappaport Vassiliadis broth (9 mL each). Aliquots (enough to fill a bacteriological loop) of the broth cultures were streaked onto XLDnov nal agar and incubated at 37℃ for 24 h.
The samples (jejunum, ileum, ileocecal valve, mesenteric lymph nodes, ileocecal lymph nodes, liver and spleen) were fixed in 10% buffered formalin. Samples were reduced to smaller pieces within 24 h of slaughter, washed in phosphate buffer saline, and processed using routine histological protocols before embedding in paraffin [26]. Serial sections 5-µm-thick were cut with a rotative microtome (Reichert, Austria), mounted on slides treated with 3-aminopropyltriethoxysilane (Sigma-Aldrich, USA), and dried in an oven at 37℃ for 24 h. To perform the histopathological evaluation, the slides were stained with hematoxylin-eosin and examined under an Olympus CH 40 microscope (Olympus, Japan). Images were captured with an Olympus 4000 digital camera (Olympus, Japan).
SWI and MLI variables were analyzed with an ANOVA using the general linear model with SPSS for Windows (ver. 11.0; SPSS, USA). Differences between mean treatment values were evaluated with Tukey's test. Results are expressed as the arithmetic mean and standard deviation. Rates of pathogen detection were analyzed using the Chi-square test with Yates correction. p-values < 0.05 were considered statistically significant.
The feces of all calves involved in the experiment were negative for Salmonella spp. both prior to the experiment and before inoculation with the pathogen. A high frequency of pathogen detection was found in target internal organs (Table 2). Salmonella detection in the internal organs was not associated with administration of the probiotic inoculum. Although Salmonella Dublin was detected in the liver from many of the infected animals, it was infrequently isolated from the bile. Additionally, the pathogen was rarely detected in popliteal lymph nodes, cerebrospinal fluid, or synovial fluid (Table 2) whereas it was commonly found in the lung and mediastinal lymph nodes.
Animals infected with Salmonella Dublin DSPV 595T had SWI values between 2 g/kg and 3 g/kg. C-G calves had an average SWI value of 3.0 g/kg while these values were 2.3 g/kg for the LAB-G group and 2.7 g/kg for the L-LAB-G animals. Although the control calves had higher SWI values than animals inoculated with the probiotics, differences between the groups were not significant.
The MLI values in target organs were 83.0%, 70.0%, and 64.3% for the C-G, LAB-G, and L-LAB-G groups, respectively. The difference between the C-G and L-LAB-G groups was significant (p < 0.05). Figs. 1A, C, 2A, C, 3A and C show some target organs after 8 h of infection. At this time, characteristic Salmonella lesions were not found. Figs. 1B, D, 2B, D, 3B and D show these characteristic lesions in certain target organs that developed after 80 h of infection. In general, the lesions were typical for Salmonella infection. However, the absence of paratyphoid nodules (PN) in the liver and spleen was evident. That is why we observed little coagulation necrosis. Microscopic lesions observed in the L-LAB-G group after 80 h of Salmonella inoculation were similar to those found in the C-G group at 32 h and 56 h post-infection. Thus, the MLI value was lower for the L-LAB-G animals.
The liver showed degenerative changes in the parenchyma with small foci of coagulation necrosis and mixed infiltrates. Proliferation of bile canaliculi was also observed along the limiting plate of the lobules (multifocal necrotizing hepatitis). Only a few animals developed acute hemorrhagic splenitis similar to that observed during any septicemic process but without PN. The gallbladder showed no signs of serious injuries in any of the groups studied.
The jejunum of control animals developed necrotizing enteritis in the mucosa that was observed during the second and fourth necropsies. Clear necrotic lesions with the presence of PN were observed in the mucosa during the fifth necropsy. In the other two groups (LAB-G and L-LAB-G), necrotizing enteritis without PN was found only after the third necropsy. The lesions were considered to be mild and non-pathognomonic.
In the ileum, necrotizing enteritis with the presence of PN in the mucosa of the control animals was found during the third and fifth necropsies. Interestingly, the lesions were least severe during the fourth necropsy. The ileum presented catarrhal enteritis with necrosis of the tip enterocytes of the villi. Mild edema of the lamina propria was also observed with casual microhemorrhages. In the LAB-G and L-LAB-G animals, lesions observed during the entire series of necropsies were minor.
Control calves developed early necrotizing enteritis (observed during the second and third necropsies) with the presence of PN as found during the fourth and fifth necropsies. In the LAB-G group, necrosis was observed only in the last three necropsies and PN were absent. In L-LAB-G animals, the PN was found only in the third necropsy. In all the other necropsies, catarrhal enteritis with edema in the lamina propria and casual microhemorrhage were noted.
Mesenteric lymph nodes in the C-G group contained PN in the cortical lymph region as observed in the second necropsy, but this was not observed in the subcapsular sinus as it normally would. In the LAB-G group, PN appeared at a later time following the fourth necropsy. In L-LAB-G animals, the same type of lesion was observed only during the third necropsy. In the ileocecal lymph nodes of C-G animals, necrotizing acute lymphadenitis with the presence of PN (subcapsular, follicular, and sinusoidal locations) was observed starting at the third necropsy. In contrast, this condition appeared after the fourth necropsy in the LAB-G and L-LAB-G groups.
In the current study, we hypothesized that the effect of an experimental probiotic inoculum on an intestinal pathogen could be measured using a model that evaluated the ability of the pathogen to translocate to the internal organs and produce lesions. In addition, we predicted that dietary lactose supplementation, together with the inoculum, would provide an advantage to animals against pathogen. Thus, we showed that the use of an experimental model of salmonellosis was useful to test the beneficial effect of a probiotic inoculum in a situation of extreme imbalance in the intestinal microbiota.
A large number of researchers have created experimental bovine models of salmonellosis using Salmonella Dublin delivered to calves through an oral route [2,6,15,17,21-23]. Overall, oral infection models that use low pathogen loads produce results more irregular, whereas high pathogen load results in the animals showing typical symptoms, a predictable evolution of the disease, that is, a model where the disease is expressed constantly and evenly. Masalski et al. [15] reported that calves inoculated with 6 × 108 CFU showed no clear clinical signs of disease and were not regular. In contrast, calves inoculated with 2 × 1010 CFU to 4 × 1010 CFU developed typical clinical signs with a more regular spread of bacteria through nasal discharges and feces. Based on this information, were considered two alternative models, each with a unique set of pros and cons, and then choose the experimental design of our study. On one hand, a model generated with low pathogen loads simulates real-world situations occurring on the farm and would thus provide more opportunities for observing the beneficial properties of the LAB experimental inoculum. However, this particular model would result in highly variable responses in the calves and any protective effects of the LAB would therefore be more difficult to measure in a small number of animals. On the other hand, a high concentration of the pathogen (improbable for naturally occurring cases of infection on farms) would create more homogeneous outcomes and therefore provide a more suitable model to measure beneficial effects of the LAB inoculum. However, this model would require the LAB inoculum to have a very high level of activity for any protective effect to be observed. Thus, the model of Salmonella infection used in our study was established taking into account this information and the fact that inoculation of 2 × 1010 CFU/animal produced a regular and homogeneous model of salmonellosis, but demanded a high level performance from the LAB inoculum to counteract the effects of the pathogen. Although it is difficult to determine the number of Salmonella that calves commonly take in while living on a farm, it is very unlikely that the bacterial concentrations are as high as those used in our experiment. The results obtained from models with high pathogen loads are more suitable for predicting the ability of a probiotic inoculum to protect against lesions caused by the pathogen.
In general, the oral entry of Salmonella, proliferation of this microorganism in the small intestine, and its rapid penetration into the lamina propria cause edema, macrophage and lymphocyte proliferation, and polymorphonuclear (PMN) recruitment. This is accompanied by expansion of the central lacteals, and generates a sharp decline in apical enterocytes while provoking a proliferative reaction in the bottom of the crypts (enteritis regenerative). This process is rapidly decompensate, causing atrophy and fusion of the villi. The bacteria also invade regional lymph nodes, leading to macrophage and PMN recruitment [25]. The same happens in submucosa veins, producing phlebitis and thromboembolism. Circulatory disorders lead to irreversible damage of the villi with apical necrosis, hemorrhage, and fibrin exudation (fibrinous necrotic enteritis). Bacteria spread through blood and the lymphatic system cause septicemia with involvement of the mesenteric lymph nodes, liver, and spleen [1,24,25]. In our study, the liver, spleen, and mesenteric and ileocecal lymph nodes (target organs) were the sites at which the pathogen was found more frequently, thus showing that Salmonella used both the lymphatic system and blood for its entry and multiplication. Target organs were also, both size and number, those with the greatest lesions.
The MLI was useful for identifying differences associated with the inoculum in the response of calves to infection with the pathogen. Lesions found in the liver and spleen were evaluated together; this is because both the spleen and liver are two target organs where specific lesions for Salmonella infection often develop. In the liver of animals living on farms, severe cholangiohepatitis with strong infiltration in the portal space is typically observed, particularly in bile canaliculi with canalicular bile stasis, a structural disorder in the lobule characterized by dilated hyperemic sinusoids and invasion of sinusoidal cells [1,12].
In calves with salmonellosis, PN appear with high regularity in lobules with an erratic distribution. In a few cases this necrotic lesion was not present, but appears the so-called thromboembolism in lobular central vein by the formation of an intravascular fibrin clot which includes pyogenic PMN [1,25]. In calves with salmonellosis, the spleen regularly develops severe hemorrhagic splenitis with randomly distributed PN foci, especially in the red pulp [1,25]. Considering the lesions found in the liver and spleen of the animals from our experiment, it could be argued that this target organs had virtually no involvement in any of the experimental group's animals, showing non-pathognomonic minor lesions (without PN). The absence of PN in spleen, possibly due peracute salmonellosis, could explain the SWI found in calves, which was similar to that reported in previous experiment in which the pathogen was not used [7]. In our experiment, lesions found in the jejunum were more moderate than those found in clinical cases observed on farms. In groups inoculated with LAB, the lesions were minor and non-pathognomonic, especially considering that Salmonella inoculation was substantial. In clinical cases of salmonellosis observed on farms, ileum lesions usually involve fibrinous necrotic enteritis and the possible presence of PN [1,12]. In the present experiment, we observed necrosis only in the control calves while lesions found during the entire series of necropsies in animals treated with probiotics were minor.
For clinical cases of salmonellosis on farms, the IV is one of the target organs (along with the surrounding lymph nodes) used for diagnosing histopathological lesions caused by the infection [1,12]. In our study, IV lesions developed early and were typical in control animals. Necrosis was found only during the first necropsy. In the subsequent necropsies, the lesions included necrosis with PN (in a very early stage of formation) while in the final necropsies the lesion found included necrosis with PN. That is, as more time passed since the administration of the pathogen, most complete, advanced and characteristic lesions were found. The results from the L-LAB-G group were encouraging because only minor lesions were found in these animals. Furthermore, lesions in the colon of all calves treated with the LAB inoculum were also minor.
Aggressiveness of the pathogenic bacteria strain and the infection time course have a marked influence on the type of lesions found in the lymph nodes in cases seen on farms. Salmonellosis caused by low virulence bacterial strains usually generate hemorrhagic type lesions in the course of several days [1,12]. When the strain is highly virulent and the infection process is hyperacute, lymph nodes are often the only organs in which PN are found (being absent in the liver and spleen). In these cases, nodules are found in the subcapsular sinus as a relatively continuous mantle of necrotic cells with pyogenic PMN in a mesh of fibrin [1,25]. In our study, PN appeared later in groups treated with the LAB inoculum than in the control animals. This should have correlated with the lesions found in the jejunum and ileum, areas into which the nodes drain. In cases on farms, lesions found in the ileocecal lymph nodes are more frequent and this is sometimes the only location where PN develop, especially in hyperacute cases [1,12].
Very few studies have examined the effect of an experimental probiotic inoculum on the ability of Salmonella to translocate to the internal organs and to produce lesions in infected calves. In the current study, administration of a probiotic inoculum was not fully effective against infection with Salmonella Dublin administered at high concentrations. Probiotic treatment was unable to delay the arrival of the pathogen to target organs. However, it was evident that the inoculum altered the response of the animals to pathogen attack because the severity of Salmonella infection was reduced and milder microscopic lesions developed in the group treated with lactose and LAB. In order to have a clearer understanding of the enhanced response of calves treated with the probiotic inoculum against pathogen, further studies of the mechanisms underlying the effect of these biotherapeutic agents should be performed.
Figures and Tables
Fig. 1
Microscopic lesions in the liver and spleen of calves from the three experimental groups: non-supplemented control (C-G), supplemented with LAB inoculum (LAB-G) at a daily dose of 109 CFU/kg body weight (BW), and supplemented with LAB inoculum at a daily dose of 109 CFU/kg BW and 100 g lactose (L-LAB-G) after infection with Salmonella Dublin DSPV 595T. (A) Liver NAL 8 h post-infection. (B) Parenchymatous degeneration with few inflammatory infiltrates without PN 80 h post-infection (liver). (C) Spleen NAL 8 h post-infection. (D) Acute hemorrhagic splenitis without PN 80 h post-infection (spleen). H&E stain, ×100.
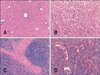
Fig. 2
Microscopic lesions in the ileum of calves from the three experimental groups: non-supplemented (C-G), supplemented with LAB inoculum at a daily dose of 109 CFU/kg BW (LAB-G), and supplemented with LAB inoculum at a daily dose of 109 CFU/kg BW and 100 g lactose (L-LAB-G) after infection with Salmonella Dublin DSPV 595T. (A) The ileum NAL 8 h post-infection. (B) The ileum with necrotizing enteritis without PN 80 h post-infection. (C) Ileal mucosa NAL 8 h post-infection. (D) Ileal mucosa with coagulative necrosis around the glandular body and mononuclear infiltrates without PN 80 h post-infection. H&E stain, ×40 (A and B), ×100 (C and D).
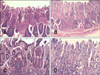
Fig. 3
Microscopic lesions in the ileocecal valve (IV) of calves from the three experimental groups: non-supplemented (C-G), supplemented with LAB inoculum at a daily dose of 109 CFU/kg BW (LAB-G), and supplemented with LAB inoculum at a daily dose of 109 CFU/kg BW and 100 g lactose (L-LAB-G) after infection with Salmonella Dublin DSPV 595T. (A) IV NAL 8 h post-infection. (B) IV with necrotizing enteritis without PN 80 h post-infection. (C) IV NAL 8 h post-infection. (D) IV with necrotizing enteritis and PN development 80 h post-infection. H&E stain, ×40.
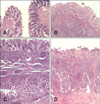
Table 1
Indicators of microscopic lesion severity used for determining the microscopic lesion index in the target organs studied

Acknowledgments
This article is dedicated to the memory of Dr. Carlos Peralta. This work was financed by the Universidad Nacional del Litoral, Santa Fe, Argentina (project CAI+D 033-229/05). The skilled technical assistance of Drs. H. I. Henzenn, E. Avataneo, A. Raspini, A. Poitevin, N. Agüero, M. O. Ferreira, and C. A. Sarchioni is gratefully acknowledged. We deeply appreciate the help received from the Calf Artificially Rearing Group at the Facultad de Ciencias Veterinarias, Universidad Nacional del Litoral, Argentina. The authors also thank the Asociación de Cooperativas Argentinas for their indispensable cooperation. The valuable help of Dr. María Inés Caffer from the Servicio de Enterobacterias del Instituto Nacional de Enfermedades Infecciosas, Administración Nacional de Laboratorios e Instituto de Salud "Dr. Carlos G. Malbrán", Argentina is acknowledged. We also wish to thank Dr. Luis Calvinho from EEA INTA Rafaela, Argentina for his support in identifying the Salmonella Dublin DSPV 595T. The authors gratefully acknowledge Mrs. Rocío Marini for the contribution to this work. Dr. L. S. Frizzo and L. P. Soto are postdoctoral fellows, and Dr. M. L. Signorini is a Research Career Member from the Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina. Dr. M. V. Zbrun is doctoral fellow from the Universidad Nacional del Litoral, Argentina.
References
1. Barker IK, Van Dreumel AA. Jubb KVF, Kennedy PC, Palmer N, editors. The alimentary system. Pathology of Domestic Animals. 1985. 3rd ed. Olando: Academic Press;160–166.

2. Deignan T, Alwan A, Kelly J, McNair J, Warren T, O'Farrelly C. Serum haptoglobin: an objective indicator of experimentally-induced Salmonella infection in calves. Res Vet Sci. 2000. 69:153–158.

3. Demecková V, Kelly D, Coutts AG, Brooks PH, Campbell A. The effect of fermented liquid feeding on the faecal microbiology and colostrum quality of farrowing sows. Int J Food Microbiol. 2002. 79:85–97.

4. Federation of Animal Science Societies. Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching. 1999. 1st ed. Savoy: Federation of Animal Science Societies;80–84.
5. Food and Agriculture Organization of the United Nations and World Health Organization. Expert Consultation Report on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. 2001. Córdoba: Food and Agriculture Organization of the United Nations and World Health Organization;4–6.
6. Forbes D, Oakley GA, Mackenzie JA. Experimental Salmonella dublin infection in calves. Vet Rec. 1977. 101:220–224.
7. Frizzo LS, Bertozzi E, Soto LP, Sequeira GJ, Rodríguez Armesto R, Rosmini MR. Studies on translocation, Acute Oral toxicity and intestinal colonization of potentially probiotic lactic acid bacteria administered during calf rearing. Livest Sci. 2010. 128:28–35.

8. Frizzo LS, Peralta C, Zbrun V, Bertozzi E, Soto LP, Marti E, Dalla Santina R, Sequeira GJ, Rosmini MR. Respuesta de ratones inoculados con bacterias lácticas de origen bovino a un desafío con Salmonella dublin. FAVE Secc Cienc Vet. 2005. 4:41–53.

9. Frizzo LS, Soto LP, Bertozzi E, Sequeira G, Marti LE, Rosmini MR. Evaluación in vitro de las capacidades probióticas microbianas orientadas al diseño de inóculos probióticos multiespecie para ser utilizados en la crianza de terneros. FAVE Secc Cienc Vet. 2006. 5:69–81.
10. Frizzo LS, Soto LP, Zbrun MV, Bertozzi E, Sequeira G, Rodríguez Armesto R, Rosmini MR. Lactic acid bacteria to improve growth performance in young calves fed milk replacer and spray-dried whey powder. Anim Feed Sci Technol. 2010. 157:159–167.

11. Frizzo LS, Soto LP, Zbrun MV, Signorini ML, Bertozzi E, Sequeira G, Rodríguez Armesto R, Rosmini MR. Effect of lactic acid bacteria and lactose on growth performance and intestinal microbial balance of artificially reared calves. Livest Sci. 2011. 140:246–252.

12. Gelberg HB. McGavin MD, Carlton WW, Zachary JF, editors. Alimentary system. Thomson's Special Veterinary Pathology. 2001. 3rd ed. St. Louis: Mosby;1–79.
13. Lee DJ, Drongowski RA, Coran AG, Harmon CM. Evaluation of probiotic treatment in a neonatal animal model. Pediatr Surg Int. 2000. 16:237–242.

14. Maia OB, Duarte R, Silva AM, Cara DC, Nicoli JR. Evaluation of the components of a commercial probiotic in gnotobiotic mice experimentally challenged with Salmonella enterica subsp. enterica ser. Typhimurium. Vet Microbiol. 2001. 79:183–189.

15. Masalski N, Belchev D, Dimitrov A, Kaloianov I, Kolev V. Experimental Salmonella dublin infection in calves. Vet Med Nauki. 1987. 24:3–10.
16. Moura LN, Neumann E, Vieira LQ, Nicoli JR. Protection by Lactobacillus acidophilus UFV-H2B20 against experimental oral infection with Salmonella enterica subsp. enterica ser. Typhimurium in gnotobiotic and conventional mice. Braz J Microbiol. 2001. 32:66–69.

17. Nazer AH, Osborne AD. Experimental Salmonella dublin infection in calves. Br Vet J. 1977. 133:388–398.
18. Rodríguez Armesto R, Peralta C, Ochoteco M, Zimmermann R, Marini R, Otero JL. Salmonelosis septicémica en terneros lactantes: nueva presentación para una vieja enfermedad. Primera parte. Revista therios. 1996. 25:251–260.
19. Rosmini MR, Sequeira GJ, Guerrero-Legarreta I, Martí LE, Dalla-Santina R, Frizzo L, Bonazza JC. Produccion de probioticos para animales de abasto: importancia del uso de la microbiota intestinal indigena. Rev Mex Ing Quim. 2004. 3:181–191.
20. Schneider R, Rosmini M, Ehrmann M, Vogel R. Identificación de bacterias lácticas componentes de la microbiota típica de los terneros criados en condiciones artificiales. FAVE Secc Cienc Vet. 2004. 3:7–15.

21. Segall T, Lindberg AA. Experimental oral Salmonella dublin infection in calves. A bacteriological and pathological study. Zentralbl Veterinarmed B. 1991. 38:169–185.

22. Smith HW, Jones JET. Observations on experimental oral infection with Salmonella dublin in calves and Salmonella choleraesuis in pigs. J Pathol Bacteriol. 1967. 93:141–156.
23. Steinbach G, Koch H, Meyer H, Klaus C. Influence of prior infection on the dynamics of bacterial counts in calves experimentally infected with Salmonella dublin. Vet Microbiol. 1996. 48:199–206.

24. Tizard IR, editor. Veterinary Immunology: an Introduction. 1996. 5th ed. Philadelphia: Saunders;154–155.
25. Von Lichtenberg F. Cotran RS, Kumar V, Robbins SL, editors. Enfermedades infecciosas. Patología Estructural y Funcional. 1990. 4th ed. Madrid: McGraw Hill-Interamericana;317–462.
26. Woods AE, Ellis RC, editors. Laboratory Histopathology: A Complete Reference. 1994. Vol. 1. New York: Churchill Livingstone;4.1, 2. 4.6, 6.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download