Abstract
The aim of this study was to assess changes of Hsp70 and HSF-1 protein and mRNA expression in stress-sensitive organs of pigs during transportation for various periods of time. Twenty pigs were randomly divided into four groups (0 h, 1 h, 2 h, and 4 h of transportation). A significant increased activity of AST and CK was observed after 1 h and 2 h of transportation. Histopathological changes in the heart, liver, and stomach indicated that these organs sustained different degrees of injury. Hsp70 protein expression in the heart and liver of transported pigs did not change significantly while it increased significantly (p < 0.05) in the stomach. Hsp70 mRNA levels decreased significantly (p < 0.05) in the heart after 4 h of transportation. However, mRNA expression increased significantly in the liver after 1 (p < 0.05) and 4 h (p < 0.01) of transportation, and increased significantly in the stomach of the transported pigs after 1, 4 (p < 0.01), and 2 h (p < 0.05). HSF-1 levels were reduced at 1 and 4 h (p < 0.05) only in the hearts of transported pigs. These results indicate that Hsp70 mediates distinct stress-related functions in different tissues during transportation.
Pigs frequently experience stress throughout life as a result of various environmental factors including weaning, transfer between enclosures, interacting with other pigs, herding, marketing, and slaughter [4]. Transport-induced stress can affect pigs in multiple ways, such as weight loss or impaired weight gain, decreased conversion of feed into meat, increased physical injury, and susceptibility to disease, which can lead to reduced meat quality [2]. Additionally, biochemical and structural changes in skeletal muscles, heart, kidney, liver, and other organs of the animals can also be caused by transport-associated stress [4,39]. Increased enzymatic activities of aminotransferase (AST) and creatine kinase (CK) in the blood serum are often associated with fatigue and muscular exercise during road transportation [24] as well as liver and heart disease [15,34]. Transport time is an important factor that affects pigs during transportation. Pérez et al. [31] compared a 15-min transport time to a 3-h transport period and found that the pigs' welfare was more compromised during the 15-min journey whereas animals that traveled for 3 h were able to better adapt to the transport conditions. Vecerek et al. [36] reported that the mortality rates during journeys under 50 km (around 1 h of transportation) was 0.06%. For journeys over 300 km (6 h or more), this rate was six-times higher at 0.34% [36].
Mammals generally respond to pathophysiological insults at the molecular level by increasing intracellular levels of heat shock proteins (Hsps) [11]. There are several families of Hsps each composed of many stress-inducible, highly conserved protein members. Hsp70 is one of the most highly conserved and strongly heat-inducible Hsps found in eukaryotic cells. Hsp70 serves as a molecular chaperone and plays a significant role in protecting cells against various cellular stressors including heat [7,10], hypoxia [9], ultraviolet irradiation [17], oxidative stress [8]. In the absence of induction of Hsps expression in cells exposed to acute stressors, the cells may undergo apoptosis or necrosis [40]. Importantly, reduced expression of Hsp70 has been reported in the kidneys and hearts of pigs after 6 h of transport stress [4]. However, the levels of Hsp70 protein and mRNA appear to vary in different tissues of pigs after being transported for different lengths of time [39].
Induction of Hsp expression in response to stress is often mediated at the transcriptional level by members of the heat shock factor (HSF) family of proteins. Mammalian genomes encode three HSF homologues: HSF-1, HSF-2, and HSF-4 [33]. In response to stress, the inactive monomeric form of HSF-1 is phosphorylated; this induces trimerization of the protein, stimulates its transactivation ability, and leads to increased expression of the Hsp genes [26]. Therefore, characterization of Hsp70 and HSF-1 expression dynamics in various tissues of pigs experiencing transport stress can provide insight into the response to transport conditions and roles of these proteins in cell-mediated protection. The aim of this study was to assess the changes of Hsp70 protein and mRNA expression in pigs transported for different periods of time, and to define the relationship between Hsp70 and HSF-1 expression in stress-sensitive organs.
All experiments were performed in accordance with the guidelines of the Animal Ethics Committee of Jiangsu province (China) and were approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University, China. During the experimental period (12 Apr. 2009), the weather was cloudy, and the temperatures were 22 ± 0.5℃ in the shelter and 24 ± 1℃ outside between 7:00 a.m. and 11:00 a.m. The relative humidity was between 68~72%. The transportation route included an equivalent mix of local roads, state roads, and highways located in Nanjing, China.
A total of 20 hybrid pigs of the Erhualian and Pietrain strains were bought from Jiangsu Academy of Agricultural Sciences (China) and raised in individual pens (2.5 × 3.0 m2) at the same place according to standard procedures. When the mean weight of the pigs reached approximately 50 ± (2) kg (at about 4 months of age), the animals were randomly divided into four groups but were kept in their individual pens until the beginning of the particular experimental transport period. The control pigs were left in their individual pens until sacrifice. The remaining three groups were transported in one commercial trailer pulled by a van (BJ1123VGJEA-A; Beiqi Foton Motor, China) containing three separate pens (5.53 × 2.00 m2, 2.21 m2 per pig) for 1, 2, or 4 h at 30~40 km/h.
After transportation, the animals in each experimental group were euthanized either in the truck or animal shelter (control group) with 3% sodium pentobarbital (10 mg/kg body weight) delivered by jugular injection, and brought to the operation center which is near to the farms. The abdomens were immediately opened and the heart, liver, and stomach were removed for later histopathological analysis. Tissue samples to be used for subsequent evaluation of Hsp expression were placed in 1.5 mL tubes and frozen in liquid nitrogen.
20 mL blood sample of each pig was immediately collected after cutting off the neck and placed on ice and subsequently centrifuged at 1,300 × g at 4℃ for 15 min. Plasma samples were stored at -20℃ until assayed. Activities of AST and CK in the serum samples were measured with commercially available kits (Nanjing Jiancheng Biochemical Reagent, China) according to the manufacturer's protocol using a clinical autoanalyzer (Vital Scientific NV, Netherlands).
Paraformaldehyde-fixed and paraffin-embedded heart, liver, and stomach tissues were cut into 4-µm serial sections, stained with hematoxylin and eosin, and examined using light microscopy (CX41; Olympus, Japan).
After washing in ice-cold physiological saline (Jiancheng Bioengineering Institute, Nanjing, China), heart, liver, and stomach samples were homogenized on ice in 10 volumes of homogenization buffer (0.15 M NaCl; 20 mM Tris-HCl, pH 8.0; 1 mM EDTA; 1 mM PMSF; 0.1 µM E-46; 0.08 µM aprotinin; 0.1 µM leupeptin; and 0.1% NP-40) using an Ultra-Turrax homogenizer (Sigma, USA). The resulting homogenates were centrifuged at 12,000 × g for 20 min at 4℃ to remove cellular debris, and the supernatants were collected and stored at -20℃ for protein quantification.
Levels of the Hsp70 and HSF-1 proteins in the heart, liver, and stomach of transport-stressed and control animals were measured using commercially available ELISA kits according to the manufacturer's instructions (QRCT-32232101 EIA\UTL, goat anti-porcine Hsp70 and QRCT-3013321031011 EIA\UTL, goat anti-porcine HSF-1; Adlitteram Diagnostic Laboratories, USA). Quantification of protein in the samples was performed by comparison to a standard curve by using these two ELISA kits. β-actin levels were also analyzed with an ELISA (sensitivity up to 0.01 ng/mL) using a commercially available kit according to the manufacturer's instructions (QRCT-3222211 EIA\UTL, goat anti-porcine β-actin; Adlitteram Diagnostic Laboratories, USA). β-actin expression was used to control for differences in total protein levels associated with the protein extraction procedure. The quantities of Hsp70 or HSF-1 in each sample were normalized using the following formula:
Relative quantity of Hsp70 (HSF-1) = quantity of Hsp70 (HSF-1) / quantity of β-actin.
After washing in ice-cold physiological saline, 0.5 g of the heart, liver, and stomach of transport-stressed and control animals were stored at -70℃ were ground in liquid nitrogen using a mortar and pestle. Total RNA was isolated from the ground tissue using TriZOL reagent (Invitrogen, USA) according to the manufacturer's instructions. RNA concentration was determined with a spectrophotometer (BMG, Germany) at a wavelength of 260 nm. 5 fold serial dilutions of RNA were prepared in ribonuclease-free water, and 2 µg of each sample was synthesized into DNA using a TRANScript Moloney murine leukemia virus (M-MLV) kit (Qiagen, Germany) according to the manufacturer's recommended protocol. The resulting cDNA was stored at -20℃ prior to use.
Complementary PCR primers were designed based on the mRNA sequences for Hsp70 [32] and GAPDH [18] obtained from the GenBank database of the National Center for Biotechnology Information (NCBI), USA. Primers were designed using Primer Premier 5.0 software (Premier Biosoft, USA). GAPDH was chosen as the internal control. Primer specificity was evaluated against the NCBI database using NCBI BLAST software. Highly purified, salt-free primers specific for Hsp70 (forward primer, 5'GCCCTGAATCCGCAGAATA-3' reverse primer, 5'TCCCCACGGTAGGAAACG-3' 152-bp product) and GAPDH (forward primer, 5'GAAGGTCGGAGTGAAC GGAT-3' reverse primer, 5'CATGGGTAGAATCATACTG GAACA-3' 149-bp product) were synthesized by Invitrogen (USA) and had an optimized annealing temperature of 60℃.
Each cDNA sample (2 µL, 25× dilution in ribonuclease-free water) was added to a reaction mixture containing 2× SYBR Premix Ex Taq (DRR041A; TaKaRa, Japan), the indicated primers (0.2 µL each), and double-distilled water in a total volume of 25 µL. FQ · RT-PCR was performed using an MX 3000P real-time PCR thermocycler (Stratagene, USA) based on the manufacturer's recommendation. Briefly, the reactions were incubated at 95℃ for 30 sec for one cycle to activate the enzyme followed by 40 cycles of denaturation at 95℃ for 5 sec, and annealing and elongation at 60℃ for 18 sec. For each run, a negative control tube lacking cDNA was processed along with the experimental samples. A five-fold dilution series of the template was used for the FQ · RT-PCR reactions to obtain two standard curves obtained with the following equations:
Y = -3.268log(x) + 24.37, r2 = 0.997 for Hsp70, and Y = -3.292log(x) + 26.43, r2 = 0.995 for GAPDH.
Amplification efficiencies of the target (Hsp70) and reference (GAPDH) mRNA sequences were about equal. Therefore, Hsp70 mRNA levels in all samples were normalized using the following formula:
Relative quantity of hsp70 mRNA = 2-ΔΔCt, where ΔΔCt = (Cthsp70 - CtGAPDH)control - (Cthsp70 mRNA - CtGAPDH)experimental.
Statistical differences between each group were assessed by a one-way analysis of variance (ANOVA) using the Statistical Package for the Social Sciences (ver. 11.5; IBM, USA). Comparisons between mean values of the control group and those of each experimental group were performed using the Duncan test for multiple comparisons. p-values less than 0.05 were regarded as statistically significant.
Significant changes in CK and AST level were observed in transported pigs compared to the control animals. CK levels were significantly increased in pigs transported for 1 and 2 h (p < 0.01) compared to the control pigs. AST levels also increased significantly after 1 (p < 0.01) and 2 h (p < 0.05) of transport compared to the controls (Table 1). However, the elevated plasma CK and AST concentrations returned to control levels after 4 h of transportation.
After 1 h of transportation, we found granular and vacuolar degeneration in the porcine liver cells (Fig. 1). More obvious vacuolar degeneration was detected in the cytoplasm of hepatocytes after 2 h of transportation, especially ones from in the center of the hepatic lobule. Hepatocyte necrosis characterized by karyolysis was also present after 4 h of transportation. No obvious lesions were found in the liver of control pigs. However nous vaculars onlyAfter 1 h of transportation, the myocardial cells showed acute granular degeneration (Fig. 2). After 2 h of transportation, finer cytoplasmic granules appeared in the cytoplasm of myocardial cells from pig hearts. Granular degeneration of the myocardial cells was identified by light pink staining, formation tiny granular particles, and loss of striations in the cytoplasm. No obvious lesions were found in the hearts of control pigs. In the stomach, a few chief cells were detached from the crest of the micosal folds after 1 h of transportation. Additionally, the capillaries in the lamina propria of the mucous membrane was filled with red blood cells. The lamina propria and submucosa tissue spaces were widened, indicative of edema. These lesions became more severe after 2 and 4 h of transportation.
Compared to control pigs, Hsp70 protein levels in the hearts of transported pigs increased slightly after 1 h of transportation. These levels were slightly reduced after 2 h of transportation, and then again increased after 4 h of transportation. However, none of these changes were significantly different relative to the controls (p > 0.05). Hsp70 mRNA expression increased slightly in the hearts of transported pigs after 1 and 2 h of transportation (p > 0.05), and then decreased after 4 h of transportation (p < 0.05; Table 2).
Hsp70 protein levels decreased continuously in the livers of transported pigs at 1, 2, and 4 h after transportation compared to the control group. However, none of these changes were significantly compared to the control animals (p > 0.05). Levels of the Hsp70 mRNA in livers of transported pigs increased at 1 h (p < 0.05) and maximized after 4 h of transportation (p < 0.01; Table 2).
Hsp70 levels in the stomachs of transported pigs were significantly increased (p < 0.05) after 1, 2, and 4 h of transportation compared to the control. Similar to the other tissues, induction of Hsp70 mRNA expression was also observed in the stomachs. However, the levels of Hsp70 transcript peaked after 2 h of transportation (Table 2).
HSF-1 expression in hearts of transported pigs decreased significantly (p < 0.05) compared to the control after 1 h of transportation. HSF-1 levels in the liver and stomach of transported pigs tended to be reduced as observed in the heart. Nevertheless, no significant differences in HSF-1 levels were observed after 1, 2, or 4 h of transportation compared to the control pigs (p > 0.05) (Fig. 3).
In the present study, CK and AST activity levels in pigs transported for various time periods were higher than those of control pigs. Increased enzymatic activities of CK and AST in the blood serum are often associated with fatigue and muscular exercise during road transportation [24] as well as liver and heart disease [15,34]. Acute degeneration was also observed in the hearts, livers, and stomachs of transported pigs. These results indicated that the heart, liver, and stomach tissues sustained damage, particularly at the beginning of transportation, and are in agreement with the findings from previous studies [5,19].
Hsps are endogenous, highly conserved proteins that play significant roles in the cellular response to stress [14,37]. Hsp70 levels in the heart found in the present study differed from those previously reported by Bao et al. [4] and Yu et al. [39]. The reason for these differences may be due to the varying levels of tolerance for transport-associated stress among pigs and piglets. Many reports have also suggested that the liver is a prime target for tissue injury resulting from environmental challenges [13,38]. The AST activity levels we observed were consistent with severe liver damage resulting from transport, particularly at the beginning of the transportation period.
Hsp70 has previously been demonstrated to have important cytoprotective functions in the gastric mucosa both in vitro and in vivo [27,28]. High levels of Hsp70 can protect gastric cells from NH2Cl-induced injury [30]. Decreased Hsp70 expression causes a reduction in gastric mucosa protection and can lead to stomach tissue injuries, including ulcers [35]. Hsp70 family members can refold degenerated proteins by recognizing and binding to the cytoskeletal myosin heavy chain and actin in damaged gastric mucosa [29]. Our results showed that the levels of the Hsp70 protein significantly increased after transportation, indicating that Hsp70 also plays an important role in protecting the stomach tissues during transportation.
In the present study, variations in Hsp70 protein levels did not correspond to changes in Hsp70 mRNA expression in the hearts, livers, or stomachs of transported pigs. These observations are not consistent with the classical transcription and translation regulatory mechanisms. However, a striking feature of heat shock genes is that expression in different organisms and types of cells within an organism can be regulated through distinct and unique mechanisms [23]. Similar to the results reported in our study, Chen et al. [6] also observed discordant Hsp70 protein and mRNA expression levels in lung adenocarcinomas. Control of Hsp70 expression appears to be regulated in a complex manner at both the transcriptional and post-transcriptional levels [20,25]. Banerji et al. [3] demonstrated that Hsp70 transcription is rapidly induced by heat shock, reaches maximal levels by 60 min, and decreases thereafter. This group also found that Hsp70 transcription increased 20-fold by 60 min and remained constant through 6 h of heat shock. These observations are similar to our findings. Cytoplasmic accumulation of Hsp70 mRNA is thought to reach a critical level that either directly or indirectly affects the rate of Hsp70 gene transcription [3]. We hypothesize that differences in the expression of Hsp70 protein and mRNA may be due to consumption of the Hsp70 protein after playing protective functions during transportation.
Induction of Hsp expression is reportedly mediated by HSF through binding to heat shock elements (HSEs) present in the promoter regions of HSP genes [12]. In the present study, HSF-1 levels varied in different tissues after transportation. Under normal conditions, HSF-1 localizes to the cytosol as an inactive monomer. However, HSF rapidly assembles into a trimer in response to heat shock or other physiological stresses and accumulates in the nucleus. Nuclear accumulation of HSF trimers leads to increased HSE binding, which activates the transcription of Hsps. HSF is then converted back into the monomeric form, and Hsp expression returns to basal levels [25]. In the present study, the levels of HSF-1 did not appear to correspond to Hsp70 expression levels in different tissues. These observations suggest that Hsp70 levels in pigs during transportation may be regulated by factors other than changes in HSF-1 expression.
A previous study showed that Hsp72, which is a number Hsp70 family, is constitutively expressed in all portions of the swine heart and that expression of this protein may not be dependent on an HSF : HSE interaction [22]. In contrast, Abravaya et al. [1] reported that transcriptional regulation of the human hsp70 gene in response to heat shock and other forms of physiological stress occurs through activation of HSF. Arimoclomol, an experimental drug that activates the heat shock response, can prolong activation of HSF-1, resulting in an increase of Hsp70 and Hsp90 expression in SOD1G93A mice [16]. However, Hsp70 can also negatively regulate HSF-1 activation [1,21]. Taken together, these results suggest that the regulatory mechanisms linking HSF-1, HSE, and Hsp70 are very complex. Whether the activation of HSF-1 was prolonged or negatively regulated by Hsp70 in the present study remains unclear and requires further examination.
Our experiments results indicate that the stress-sensitive organs (heart, liver and stomach) of pigs sustained different degrees of injury during transportation. Hsp70 mediates distinct stress-related functions in different tissues during transportation.
Figures and Tables
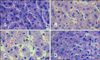 | Fig. 1Representative images of hepatocytes from control and transported pigs. (A) Hepatocytes of a non-transported (control) pig. (B) Obvious granular and vacuolar degeneration characterized by increased cell size, faint and lightly stained cytoplasm, and expanded intracellular spaces (arrow) was observed in the cytoplasm of hepatocytes from pigs transported for 1 h. (C) Enlarged granular and vacuolar degeneration (arrow) in the cytoplasm of hepatocytes from pigs transported for 2 h. (D) Obvious necrotic hepatocytes characterized by karyopyknosis and karyolysis (arrow) in the hepatic lobule of pigs transported for 4 h. H&E stain. Scale bars = 10 µm. |
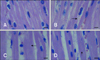 | Fig. 2Representative images of myocardial cells from control and transported pigs. (A) Myocardial fibers in a non-transported (control) pig. (B) Granular degeneration (arrow) in the cytoplasm of myocardial fibers from a pig after 1 h of transportation. (C) Granular degeneration of myocardial cells indicated by light pink stpaining, tiny granular particles, and loss of striations in the cytoplasm (arrow) after 2 h of transportation. (D) Acute exudation characteristic of granular degeneration (arrow) among myocardial cells from pigs after 4 h of transportation. H&E stain. Scale bars = 10 µm. |
 | Fig. 3HSF-1 protein levels in the heart, liver, and stomach of transported pigs. *p < 0.05 and **p < 0.01 compared to the 0 h. Values are presented as the mean ± SD. |
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 30972165), the National Department Public Benefit Research Foundation (Agriculture, No. 201003060-11), Natural Science Foundation of Jiangsu Province (No. BK2008059), China; the Sino-German Agricultural Cooperation Project of the Federal Ministry of Food, Agriculture, and Consumer Production, Germany; A Project Funded by the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions and the Nature Science Fund for Colleges and Universities in Jiangsu Province (No. 11KJD230001), China.
References
1. Abravaya K, Myers MP, Murphy SP, Morimoto RI. The human heat shock protein hsp70 interacts with HSF, the transcription factor that regulates heat shock gene expression. Genes Dev. 1992. 6:1153–1164.

2. Adeola O, Ball RO. Hypothalamic neurotransmitter concentrations and meat quality in stressed pigs offered excess dietary tryptophan and tyrosine. J Anim Sci. 1992. 70:1888–1894.

3. Banerji SS, Berg L, Morimoto RI. Transcription and post-transcriptional regulation of avian HSP70 gene expression. J Biol Chem. 1986. 261:15740–15745.

4. Bao E, Sultan KR, Nowak B, Hartung J. Expression and distribution of heat shock proteins in the heart of transported pigs. Cell Stress Chaperones. 2008. 13:459–466.

5. Bao ED, Sultan KR, Nowak B, Hartung J. Localization and expression of heat shock proteins in liver of transport stressed pigs. Zhongguo Nong Ye Ke Xue. 2002. 35:1130–1133.
6. Chen G, Gharib TG, Huang CC, Taylor JMG, Misek DE, Kardia SLR, Giordano TJ, Iannettoni MD, Orringer MB, Hanash SM, Beer DG. Discordant protein and mRNA expression in lung adenocarcinomas. Mol Cell Proteomics. 2002. 1:304–313.

7. Cvoro A, Dundjerski J, Trajković D, Matić G. Heat stress affects the glucocorticoid receptor interaction with heat shock protein Hsp70 in the rat liver. Biochem Mol Biol Int. 1998. 46:63–70.

8. Drummond IAS, Steinhardt RA. The role of oxidative stress in the induction of Drosophila heat-shock proteins. Exp Cell Res. 1987. 173:439–449.

9. Dwyer BE, Nishimura RN, Brown IR. Synthesis of the major inducible heat shock protein in rat hippocampus after neonatal hypoxia-ischemia. Exp Neurol. 1989. 104:28–31.

10. Evdonin AL, Guzhova IV, Margulis BA, Medvedeva ND. Extracellular heat shock protein 70 mediates heat stress-induced epidermal growth factor receptor transactivation in A431 carcinoma cells. FEBS Lett. 2006. 580:6674–6678.

11. Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999. 61:243–282.

12. Fujikake N, Nagai Y, Popiel HA, Kano H, Yamaguchi M, Toda T. Alternative splicing regulates the transcriptional activity of Drosophila heat shock transcription factor in response to heat/cold stress. FEBS Lett. 2005. 579:3842–3848.

13. Granger DN, Höllwarth ME, Parks DA. Ischemia-reperfusion injury: role of oxygen-derived free radicals. Acta Physiol Scand Suppl. 1986. 548:47–63.
14. Gullo CA, Teoh G. Heat shock proteins: to present or not, that is the question. Immunol Lett. 2004. 94:1–10.

15. Jabiry-Zieniewicz Z, Bobrowska K, Kaminski P, Wielgos M, Zieniewicz K, Krawczyk M. Low-dose hormonal contraception after liver transplantation. Transplant Proc. 2007. 39:1530–1532.

16. Kieran D, Kalmar B, Dick JRT, Riddoch-Contreras J, Burnstock G, Greensmith L. Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nat Med. 2004. 10:402–405.

17. Kwon SB, Young C, Kim DS, Choi HO, Kim KH, Chung JH, Eun HC, Park KC, Oh CK, Seo JS. Impaired repair ability of hsp70.1 KO mouse after UVB irradiation. J Dermatol Sci. 2002. 28:144–151.
18. Li HM, Niki T, Taira T, Iguchi-Ariga SMM, Ariga H. Association of DJ-1 with chaperones and enhanced association and colocalization with mitochondrial Hsp70 by oxidative stress. Free Radic Res. 2005. 39:1091–1099.

19. Li Y, Sun P, Wang Z, Bao ED. Relationship between pathological lesion and distribution of HSP27 in transport stressed pigs. Nanjing Nong Ye Da Xue Xue Bao. 2005. 28:83–87.
21. Locke M, Tanguay RM. Increased HSF activation in muscles with a high constitutive Hsp70 expression. Cell Stress Chaperones. 1996. 1:189–196.

22. Locke M, Tanguay RM, Ianuzzo CD. Constitutive expression of HSP 72 in swine heart. J Mol Cell Cardiol. 1996. 28:467–474.

23. McGarry TJ, Lindquist S. The preferential translation of Drosophila hsp70 mRNA requires sequences in the untranslated leader. Cell. 1985. 42:903–911.

24. Miranda-de la Lama GC, Rivero L, Chacón G, Garcia-Belenguer S, Villarroel M, María GA. Effect of the pre-slaughter logistic chain on some indicators of welfare in lambs. Livest Sci. 2010. 128:52–59.

25. Morimoto RI. Cells in stress: transcriptional activation of heat shock genes. Science. 1993. 259:1409–1410.

26. Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998. 12:3788–3796.

27. Nakamura K, Rokutan K, Marui N, Aoike A, Kawai K. Induction of heat shock proteins and their implication in protection against ethanol-induced damage in cultured guinea pig gastric mucosal cells. Gastroenterology. 1991. 101:161–166.

28. Odashima M, Otaka M, Jin M, Konishi N, Sato T, Kato S, Matsuhashi T, Nakamura C, Watanabe S. Induction of a 72-kDa heat-shock protein in cultured rat gastric mucosal cells and rat gastric mucosa by zinc L-carnosine. Dig Dis Sci. 2002. 47:2799–2804.
29. Otaka M, Odashima M, Tamaki K, Watanabe S. Expression and function of stress (heat shock) proteins in gastrointestinal tract. Int J Hyperthermia. 2009. 25:634–640.

30. Oyake J, Otaka M, Matsuhashi T, Jin M, Odashima M, Komatsu K, Wada I, Horikawa Y, Ohba R, Hatakeyama N, Itoh H, Watanabe S. Over-expression of 70-kDa heat shock protein confers protection against monochloramine-induced gastric mucosal cell injury. Life Sci. 2006. 79:300–305.

31. Pérez MP, Palacio J, Santolaria MP, Aceña MC, Chacón G, Gascón M, Calvo JH, Zaragoza P, Beltran JA, García-Belenguer S. Effect of transport time on welfare and meat quality in pigs. Meat Sci. 2002. 61:425–433.

32. Pilon N, Daneau I, Paradis V, Hamel F, Lussier JG, Viger RS, Silversides DW. Porcine SRY promoter is a target for steroidogenic factor 1. Biol Reprod. 2003. 68:1098–1106.
33. Pirkkala L, Nykänen P, Sistonen L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 2001. 15:1118–1131.

34. Radosavljević T, Mladenović D, Vučević D, Petrović J, Hrnčić D, Djuric D, Lončar-Stevanović H, Stanojlović O. Effect of acute lindane and alcohol intoxication on serum concentration of enzymes and fatty acids in rats. Food Chem Toxicol. 2008. 46:1739–1743.

35. Shichijo K, Ihara M, Matsuu M, Ito M, Okumura Y, Sekine I. Overexpression of heat shock protein 70 in stomach of stress-induced gastric ulcer-resistant rats. Dig Dis Sci. 2003. 48:340–348.
36. Vecerek V, Grbalova S, Voslarova E, Janackova B, Malena M. Effects of travel distance and the season of the year on death rates of broilers transported to poultry processing plants. Poult Sci. 2006. 85:1881–1884.

37. Whitley D, Goldberg SP, Jordan WD. Heat shock proteins: a review of the molecular chaperones. J Vasc Surg. 1999. 29:748–751.

38. Ye X, Meeker HC, Kozlowski PB, Wegiel J, Wang KC, Imaki H, Carp RI. Pathological changes in the liver of a senescence accelerated mouse strain (SAMP8): a mouse model for the study of liver diseases. Histol Histopathol. 2004. 19:1141–1151.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download