Abstract
The incidence of diabetes mellitus is increasing among companion animals. This disease has similar characteristics in both humans and animals. Diabetes is frequently identified as an independent risk factor for infections associated with increased mortality. In the present study, homozygous diabetic (db/db) mice were infected with Listeria (L.) monocytogenes and then treated with the anti-diabetic drug exendin-4, a glucagon-like peptide 1 analogue. In aged db/db mice, decreased CD11b+ macrophage populations with higher lipid content and lower phagocytic activity were observed. Exendin-4 lowered high lipid levels and enhanced phagocytosis in macrophages from db/db mice infected with L. monocytogenes. Exendin-4 also ameliorated obesity and hyperglycemia, and improved ex vivo bacteria clearance by macrophages in the animals. Liver histology examined during L. monocytogenes infection indicated that abscess formation was much milder in exendin-4-treated db/db mice than in the control animals. Moreover, mechanistic studies demonstrated that expression of ATP binding cassette transporter 1, a sterol transporter, was higher in macrophages isolated from the exendin-4-treated db/db mice. Overall, our results suggest that exendin-4 decreases the risk of infection in diabetic animals by modifying the interaction between intracellular lipids and phagocytic macrophages.
The World Health Organization predicts that by 2030 there will be more than 360 million diabetic patients worldwide, and has declared that diabetes is an alarming epidemic [38]. Diabetes mellitus is also the most frequently diagnosed endocrinopathy in cats and dogs [31]. Classification of diabetic animals is modeled after the human classification. Type 1 diabetes mellitus, characterized by insulin dependency, is most common in dogs whereas non-insulin-dependent type 2 diabetes mellitus appears to be the dominant form of diabetes in cats [15,31]. Obesity is a well-established risk factor for type 2 diabetes in both felines and humans [9,28] but not in dogs [31]. However, some studies have indicated that obese dogs fed a high-fat diet will develop insulin resistance [27,39].
Lipid bodies are dynamic organelles that are involved in the innate host immune response to pathogen infection [26]. Macrophages accumulate lipoproteins to form intracellular lipid bodies which become lipid-laden macrophages. These lipid-laden macrophages have decreased phagocytic capacity and disrupted cytoskeletons [5].
Listeria (L.) monocytogenes is an intracellular bacterium that causes opportunistic infections in many immunocompromised populations. Pregnant animals and their fetuses are at highest risk of developing listeriosis as are human infants, the elderly, and immunocompromised patients including diabetics [36]. Listeriosis has been recognized as an important food-borne disease among humans and many outbreaks are attributed to contaminated milk, poultry, and livestock products [34,35]. Listeria is also an infectious pathogen that is transmitted from dogs and cats to humans [25]. The homozygous diabetic (db/db) mouse, a model for diabetic dyslipidemia, has impaired host resistance to L. monocytogenes. Diabetic patients have also been frequently identified as a population with a high risk for bacterial infection [20]. Furthermore, db/db mice fed a Western diet have plasma lipoprotein levels similar to those of human patients with type 2 diabetes mellitus [22].
Currently, there are no anti-diabetic drugs that can improve host defense against L. monocytogenes by decreasing macrophage lipid content. Exendin-4 (Byetta), a new generation of anti-diabetic drug, is a glucagon-like peptide 1 (GLP-1) analogue that decreases lipid accumulation in diabetic patients by stimulating insulin secretion and increasing insulin sensitivity [33]. To our knowledge, no reports have indicated that GLP-1 analogues can influence lipid metabolism or related phagocytic activity of macrophages. Many type 2 diabetic animals are also obese (a condition sometimes called "diabesity"), and identifying single compounds for simultaneously treating these conditions is a challenge [1]. Exendin-4 is a potential candidate due to its ability to stimulate insulin secretion and induce weight loss while incurring a minimal risk of hypoglycemia [1,2]. Moreover, exendin-4 has an anti-diabetic effect on db/db mice [7,40].
In the present study, we measured the lipid content of macrophages in db/db mice after exendin-4 administration. We found that macrophages from mice treated with exendin-4 had lower lipid levels and higher phagocytic activity than ones from control animals. We also demonstrated that exendin-4 was able to enhance resistance to L. monocytogenes infection in db/db mice. Moreover, exendin-4 increased the expression of ATP binding cassette transporter 1 (ABCA1) that facilitated cholesterol efflux from lipid-laden macrophages in the mice.
Exendin-4 was purchased from Sigma (USA). L. monocytogenes (BCRC 15386) was obtained from the Bioresource Collection and Research Center (Taiwan). Homozygous diabetic (db/db) C57BL/KsJ mice and non-diabetic control littermates (db/m) were purchased from the Jackson Laboratory (USA). All the animals were maintained in an institutional animal facility of National Chung Hsing University (Taichung, Taiwan) and handled according to the guidelines of the Institutional Animal Care and Utilization Committee, National Chung Hsing University.
Six-week-old female db/db mice were given intraperitoneal injections of exendin-4 (10 µg/kg body weight) or an equivalent volume of PBS twice per day for various periods of time. Blood glucose levels from tail vein blood were measured using an Elite glucometer (Bayer, USA) during 8:00 a.m.~9:00 a.m. Total cholesterol (TC), high-density lipoprotein (HDL), and triglyceride (TG) levels in the serum were measured using a Spotchem EZ SP-4430 (Arkray, Japan). Low-density lipoprotein (LDL) concentrations were calculated with the formula: TC - HDL - TG/5.
For PEC preparation, 6~10 mL of cold sterile PBS was injected into the peritoneal cavity. Resident exudate macrophages from the mice were harvested by peritoneal lavage, followed by centrifugation. Each experimental group included 5~6 female db/db mice. The adherent PECs were counted, stained with specific anti-mouse CD11b-PE (BioLegend, USA) or ABCA1-fluorescein isothiocyanate (FITC) (Abcam, UK), and analyzed with a fluorescence-activated cell sorter (FACS) (Coulter Epics XL-MCL; Beckman Coulter, USA). CD11b is one of macrophage markers and ABCA1 is a membrane protein that mediates cholesterol export from macrophages. PECs in the same batch were stained with Nile red (Molecular Probes, USA) and Oil Red O (Sigma-Aldrich, USA), and then examined under a light microscope (Eclipse 50i; Nikon, Japan).
Adherent PECs were collected to measure phagocytic activity. Dextran 40-FITC (1 mg/mL; Sigma, USA) was added to Dulbecco's modified Eagle's medium (DMEM) (GIBCO, USA) for 30~60 min. PECs were washed twice with FACS buffer (PBS supplemented with 0.1% FBS) and once with FACS fixative buffer (2% paraformaldehyde in FACS buffer), and analyzed with FACS. Adherent PECs (2 × 105 cells) collected from db/db mice treated with or without exendin-4 were incubated with L. monocytogenes [5 × 106 colony forming units (CFUs)] in DMEM without antibiotics for 30 min at 37℃. The extracellular bacteria were collected from supernatant after centrifuging PEC under L. monocytogenes infections. The pellets were lysed in 1 mL sterile ice-cold water to collect intracellular bacteria. The numbers of intracellular and extracellular bacteria were analyzed by performing a CFU assay with trypticase soy agar (Neogen, USA).
For the protection study of exendin-4 on Listeria-infected diabetic mice, db/db mice were injected twice daily with PBS or exendin-4 (10 µg/kg body weight) from 6 weeks to 10 or 20 weeks of age. Liver samples from the control and exendin-4-treated db/db mice were collected 48 h after intraperitoneal delivery of 5 × 104 CFU L. monocytogenes in 1 mL sterile PBS. All these samples were collected from mice at 1~2 h after final PBS or exendin-4 administration. Five µm sections of the liver were cut, stained with hematoxylin and eosin or Gram stain, and observed with a light microscope.
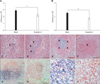
The total number of PECs was higher in db/db mice than in db/m mice. However, the percentage of CD11b+ macrophages among the PECs from db/db mice was lower than that observed in db/m mice, especially among the 24-week-old animals (Fig. 1A). The absolute number of CD11b+ macrophages among the PECs of 6-week-old db/db mice was higher than those in db/m mice and older db/db animals (Fig. 1A).
The number of lipid-laden PECs was increased in db/db mice as compared to db/m mice. Different levels of PEC lipids were observed among db/db mice of different ages. The lipid levels of PEC from 44-week-old db/db mice were higher than the ones from 6-week-old db/db mice (Fig. 1B).
The phagocytic activity of adherent PECs from db/m mice (60%) was greater than that of ones from db/db mice (< 50%). The level of PEC phagocytosis in younger db/db mice (~50%) was greater than that observed in older db/db mice (~30%; Fig. 1C). Macrophages containing high levels of lipid showed diminished phagocytic ability.
PECs collected from exendin-4-treated db/db mice had lower levels of lipid than those recovered from the untreated control animals (Fig. 2A). Macrophages less laden with lipids were also observed among PECs collected from exendin-4-treated db/db mice and had higher levels of phagocytic activity (Fig. 2B). Additionally, we observed higher ABCA1 expression in PECs from exendin-4-treated db/db mice than those from control mice (Fig. 2C).
Exendin-4 can stimulate islet β cells to secret insulin, thereby improving insulin sensitivity in diabetic patients or mice while reducing obesity. Exendin-4-treated db/db mice aged 8 to 18 weeks had a lower percentage of body weight gain (44%) compared to the control db/db mice (48%). Although our administrative dosage of exendin-4 to db/db mice was lower than previous studies, blood glucose levels of the db/db mice treated with exendin-4 were significantly lower than those of the control animals. Of particular note, hyperglycemic lesions did not deteriorate in 14-week-old and 18-week-old db/db mice after exendin-4 treatment as compared with 6-week-old db/db mice (Fig. 3A). Significantly lower levels of blood TG, TC, and LDL were observed in 12-week-old db/db mice treated with exendkn-4 compared to the control mice (Figs. 3B~E).
Extracellular L. monocytogenes populations in the exendin-4 treated group were significantly lower than those in the db/db control group, indicating that PECs from the exendin-4-treated db/db mice had higher levels of phagocytic activity (Fig. 4A). Intracellular L. monocytogenes populations in the exendin-4-treated db/db mice were also significantly lower than those in the control animals. These results suggest that exendin-4 might enhance the ability of PECs to clear bacteria (Fig. 4B).
Hydropic degeneration and fatty changes in hepatocytes were more severe in control db/db mice than exendin-4-treated db/db animals regardless of age (data not shown). Lipid quality and content are known to influence the phagocytic activity of macrophages. Not surprisingly, pathological changes in the livers of L. monocytogenes-infected control and exendin-4 treated db/db mice were observed. Mononuclear leukocytes infiltrates were more prominent in the Listeria-infected livers of exendin-4-treated db/db compared to the control animals (Figs. 4C and D). At the same time, abscess formation and necrotic lesions were decreased in the mice given exendin-4 (Figs. 4E and F). We also confirmed that Listeria populations decreased in the hepatic microabscesses of exendin-4-treated db/db mice (Figs. 4G~J).
It is well known that patients with type 1 and type 2 diabetes mellitus are at increased risk for infection with different pathogens [8,20]. Both clinical and in vitro studies of leukocyte function have indicated that infection is more likely in diabetics when blood glucose levels are poorly controlled in animals and humans [4,12,20]. It is still unclear whether or not energy utilization in phagocytes from diabetic patients influences host defense. In the present study, the number of CD11b+ macrophages among the PECs decreased in older db/db mice. Macrophage lipid levels were greater in db/db mice compared to the non-diabetic db/m mice. Concurrently, phagocytic activity of macrophages from db/db mice decreased, especially that from older animals, compared to ones from the db/m mice. These results suggest that macrophages with high lipid levels are able to phagocytize less efficiently and provide a possible mechanism of lower resistance to Listeria infection in diabetic patients. Listeriolysin (LLO), a cholesterol-dependent pore-forming cytolysin [29], is a factor necessary for listerial infection [32]. It is possible that abnormal lipid metabolism may modify the interaction between listerial LLO and macrophages in diabetic patients, thus making diabetics a high risk population for L. monocytogenes infection [36].
Exendin-4 also enhanced ex vivo Listeria clearance by macrophages among the PECs from db/db mice in our study. Simultaneous decrease of Listeria CFUs from the extracellular and intracellular compartments of macrophages from exendin-4-treated animals indicated that the drug promoted phagocytic activity and bacteria clearance. Therefore, more experiments will be necessary to further study the immunoregulatory effects of exendin-4 on macrophages and other leukocytes, except the lipid efflux activity of exendin-4 works on macrophages to enhance bacteria clearance.
Lower degrees of mononuclear cells infiltration along with more hydropic degeneration of hepatocytes and hepatic abscess formation after L. monocytogenes infection have been observed in db/db mice compared to db/m mice [18]. In the present study, exendin-4-treated db/db mice showed improved fatty liver changes at various ages (data not shown) and amelioration of hepatic lesions, including ones due to hydropic degeneration and abscess formation. Moreover, Gram staining indicated that exendin-4-treated db/db mice had milder Listeria infiltration in the liver than the control db/db animals.
Studies of genetic factors indicated that type 2 diabetes is the most common form of the disease in cats. In contrast, half of diabetic dogs have type 1 diabetes and express antibodies against islet β cells [15,31]. Overrepresented dog leukocyte antigen gene influences the type 1 diabetes expression, especially on Samoyed dogs and Miniature Poodles [16,31]. Companion animals eat energy-dense diets that induce obesity-related hyperglycemia and impaired glucose tolerance which in turn increase the risk of infection [21,31,37].
L. monocytogenes is an intracellular bacterium that is ordinarily harmless to healthy animals. However, activated macrophages are the major effector cells in hosts resistant to L. monocytogenes infection [18]. Phagocytic activity of macrophages from diabetic patients is reduced [23]. Moreover, the phagocytic capacity of lipid-laden macrophages is decreased with rapid reorganization of the actin cytoskeleton [5]. Unfortunately, similar phagocytic assays were not conducted in the present study on the db/m and db/db mice at different ages, but the diabetic mice showed poor pathogen clearance during infection with the bacteria. Hyperglycemia also induces inefficient elimination of L. monocytogenes from the liver of db/db mice [18]. We thus believe that as diabetic patients age, scavenger receptors and the lipid efflux system are modified to compensate for impaired host immunity and decreased macrophage phagocytic activity.
GLP-1 analogues and dipeptidyl peptidase IV inhibitors, both belonging to a new generation of anti-diabetic drugs, increase blood GLP-1 concentrations or increase GLP-1 half-life, respectively. These effects stimulate insulin secretion, increases insulin sensitivity, counter obesity, and decrease body lipid accumulation in diabetic patients [10]. Moreover, the insulin release levels of exogenous infusion of systemic glucose and GLP-1 followed by glucose stimulation were similar in dogs, suggesting an indirect mechanism of GLP-1 action [19]. Pharmacokinetic analysis of GLP-1 in beagles showed that the plasma half-life of GLP-1 is very short and the first-phase is less than 2.5 min [30]. GLP-1 is useful for treating diabetic cats because of its stimulatory effect on insulin secretion and synthesis by islet β cells [24]. Furthermore, significant differences in glucose, insulin, and GLP-1 responses after oral glucose stimulation have been observed between lean and obese cats [17]. Exenatide, a GLP-1 mimetic reagent, affects insulin secretion in cats in a glucose-dependent manner, similar to human and rodent [11]. This compound persists for 8 h after injection in cat which shows prolonged half-life in vivo as compared to the one of endogenous GLP-1 [11].
Exendin-4 has been shown to lower glucose levels and increase insulin sensitivity in several diabetic animal models, including db/db mice [7,40]. However, there have been no reports to date about the impact of exendin-4 on the association between lipid metabolism and phagocytic activity of macrophages in diabetic humans or mice. In the current study, we showed that exendin-4 decreased lipid levels and enhance phagocytosis in macrophages from db/db mice. Scavenger receptors and lipid efflux channel expression are known to influence intracellular lipid content and phagocytosis in macrophages [3]. The peritoneal macrophages we recovered from the db/db mice showed significantly increased expression of CD36 and SR-A, two scavenger receptors, which directly mediate cholesteryl ester accumulation. Notably, peritoneal macrophages from db/m and db/db mice have been found to perform equivalent levels of fluid-phase endocytosis and large particle phagocytosis [13].
Our short-term study did not include a lipid content analysis or measure the relative phagocytic activity of macrophages from db/db mice of different ages. Changes in the saturated/unsaturated fatty acid ratio, one measurement of membrane fluidity, can influence macrophage adhesion and phagocytic activity [6]. Previous studies have indicated that ABCA1 is highly regulated in macrophages, and controls the efflux of cholesterol and phospholipids into apolipoproteins [14]. Macrophage ABCA1 contributes to HDL formation; however, the effect on total plasma HDL levels is minimal [14]. Our experiments showed that ABCA1 expression was higher in macrophages from exendin-4 treated db/db mice than in the control db/db mice. This may have been associated with decreased lipid content and enhanced phagocytosis in the macrophages from the control animals.
In conclusion, higher lipid levels in macrophages resulted in decreased phagocytic activity. We found that exendin-4 can be used to treat hepatic lesions and enhance resistance to L. monocytogenes infection in db/db mice. These activities might suppress the effects of hyperglycemia and hyperlipidemia on diabetic patients with impaired immune defense systems. Moreover, our findings demonstrated that ABCA1 could be involved in lipid metabolism in macrophages and influence listerial resistance. Taken together, data from this study suggest that exendin-4 is suitable for use in diabetic patients in order to prevent and treat bacterial infections.
Figures and Tables
Fig. 1
Levels of CD11b+ expression, lipid, and phagocytic activity in peritoneal exudate cells (PECs) isolated from db/m mice and db/db mice at the indicated ages. (A) These cells were stained with specific anti-mouse CD11b-PE and analyzed with fluorescence-activated cell sorter (FACS). The fraction in each plot represents the total number of PECs /number of CD11b cells. (B) PECs were stained with Nile red and analyzed with FACS. Inserted panels show PECs stained with Oil Red O to identify lipid-laden macrophages. (C) Phagocytic efficacy of PECs isolated from db/m mice and db/db mice were incubated with dextran 40-fluorescein isothiocyanate (DX40-FITC) and analyzed with FACS. db/db: homozygous diabetic mice, db/m: non-diabetic control littermates, wk: week-old.

Fig. 2
Effects of exendin-4 on lipid contents, phagocytic activity, and ABCA1 expression in macrophages. (A) Adherent PECs were stained with Nile red to detect intercellular lipid and analyzed with FACS. (B) DX40-FITC was phagocytized by adherent PECs and the cells were analyzed with FACS. (C) The PECs shown in panel (A) were also stained with specific anti-mouse ABCA1-FITC and analyzed with FACS.

Fig. 3
Analysis of blood from control and exendin-4 treated db/db mice. The blood glucose (A), triglyceride (B), total cholesterol (C), HDL (D) and LDL (E) levels of db/db mice treated with or without exendin-4 were measured when the mice were various ages (n = 3 for both groups). Results are expressed as the mean ± SD. Statistically significant differences between the control (mock) and exendin-4-treated db/db mice are indicated by an asterisk (*p < 0.05). HDL: high-density lipoprotein, LDL: low-density lipoprotein.

Fig. 4
Ex vivo Listeria (L.) monocytogenes clearance by macrophages and examination of livers from exendin-4 treated db/db mice. (A) CFU values of supernatants of DMEM contained Listeria-infected macrophages were determined using the plate method with trypticase soy agar (TSA) plate. (B) The number of L. monocytogene CFUs among centrifuged pellets of macrophages were lysed in sterile cold water and determined using the plate method with TSA plate. Results are expressed as the mean ± SD. Statistically significant differences between control (mock) and exendin-4 treated db/db mice are indicated by an asterisk (*p < 0.05). Liver sections from the control (C and E) and exendin-4-treated (D and F) db/db mice were obtained 48 h after L. monocytogenes infection. Mononuclear cell infiltrates (arrowheads) were more prominent in the exendin-4-treated db/db mice than the control animals. Abscess formation (arrowheads) was much milder in the exendin-4-treated animals than in the control. Listeria was more widely distributed in the hepatic abscesses of the control db/db mice (G) than ones treated with exendin-4 (H). (I and J) The magnified areas of panels G and H. H&E (A~E) and Gram (G~J) stain. Scale bars = 100 µm.
Acknowledgments
This work was supported by grants from the Taiwan National Science Council (Nos. 97-2320-B-005-001-MY3 and 98-2815-C-005-081-B), Taiwan.
References
2. Barber TM, Begbie H, Levy J. The incretin pathway as a new therapeutic target for obesity. Maturitas. 2010. 67:197–202.

3. Bared SM, Buechler C, Boettcher A, Dayoub R, Sigruener A, Grandl M, Rudolph C, Dada A, Schmitz G. Association of ABCA1 with syntaxin 13 and flotillin-1 and enhanced phagocytosis in tangier cells. Mol Biol Cell. 2004. 15:5399–5407.

4. Bennett N. Monitoring techniques for diabetes mellitus in the dog and the cat. Clin Tech Small Anim Pract. 2002. 17:65–69.

5. Berfield AK, Abrass CK. IGF-1 induces foam cell formation in rat glomerular mesangial cells. J Histochem Cytochem. 2002. 50:395–403.

6. Calder PC, Bond JA, Harvey DJ, Gordon S, Newsholme EA. Uptake and incorporation of saturated and unsaturated fatty acids into macrophage lipids and their effect upon macrophage adhesion and phagocytosis. Biochem J. 1990. 269:807–814.

7. Chen D, Liao J, Li N, Zhou C, Liu Q, Wang G, Zhang R, Zhang S, Lin L, Chen K, Xie X, Nan F, Young AA, Wang MW. A nonpeptidic agonist of glucagon-like peptide 1 receptors with efficacy in diabetic db/db mice. Proc Natl Acad Sci USA. 2007. 104:943–948.

8. Clements RS Jr, Bell DSH. Complications of diabetes. Prevalence, detection, current treatment, and prognosis. Am J Med. 1985. 79(5A):2–7.

9. Crenshaw KL, Peterson ME. Pretreatment clinical and laboratory evaluation of cats with diabetes mellitus: 104 cases (1992-1994). J Am Vet Med Assoc. 1996. 209:943–949.
10. DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005. 28:1092–1100.

11. Gilor C, Graves TK, Gilor S, Ridge TK, Rick M. The GLP-1 mimetic exenatide potentiates insulin secretion in healthy cats. Domest Anim Endocrinol. 2011. 41:42–49.

12. Glass EJ, Stewart J, Weir DM. Altered immune function in alloxan-induced diabetes in mice. Clin Exp Immunol. 1986. 65:614–621.
13. Guest CB, Hartman ME, O'Connor JC, Chakour KS, Sovari AA, Freund GG. Phagocytosis of cholesteryl ester is amplified in diabetic mouse macrophages and is largely mediated by CD36 and SR-A. PLoS One. 2007. 2:e511.

14. Haghpassand M, Bourassa PAK, Francone OL, Aiello RJ. Monocyte/macrophage expression of ABCA1 has minimal contribution to plasma HDL levels. J Clin Invest. 2001. 108:1315–1320.

15. Hoenig M. Comparative aspects of diabetes mellitus in dogs and cats. Mol Cell Endocrinol. 2002. 197:221–229.

16. Hoenig M, Dawe DL. A qualitative assay for beta cell antibodies. Preliminary results in dogs with diabetes mellitus. Vet Immunol Immunopathol. 1992. 32:195–203.

17. Hoenig M, Jordan ET, Ferguson DC, de Vries F. Oral glucose leads to a differential response in glucose, insulin, and GLP-1 in lean versus obese cats. Domest Anim Endocrinol. 2010. 38:95–102.

18. Ikejima S, Sasaki S, Sashinami H, Mori F, Ogawa Y, Nakamura T, Abe Y, Wakabayashi K, Suda T, Nakane A. Impairment of host resistance to Listeria monocytogenes infection in liver of db/db and ob/ob mice. Diabetes. 2005. 54:182–189.

19. Ionut V, Liberty IF, Hucking K, Lottati M, Stefanovski D, Zheng D, Bergman RN. Exogenously imposed postprandial-like rises in systemic glucose and GLP-1 do not produce an incretin effect, suggesting an indirect mechanism of GLP-1 action. Am J Physiol Endocrinol Metab. 2006. 291:E779–E785.

20. Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med. 1999. 341:1906–1912.

21. Kaiyala KJ, Prigeon RL, Kahn SE, Woods SC, Porte D Jr, Schwartz MW. Reduced β-cell function contributes to impaired glucose tolerance in dogs made obese by high-fat feeding. Am J Physiol Endocrinol Metab. 1999. 277:E659–E667.
22. Kobayashi K, Forte TM, Taniguchi S, Ishida BY, Oka K, Chan L. The db/db mouse, a model for diabetic dyslipidemia: molecular characterization and effects of Western diet feeding. Metabolism. 2000. 49:22–31.

23. Lecube A, Pachón G, Petriz J, Hernández C, Simó R. Phagocytic activity is impaired in type 2 diabetes mellitus and increases after metabolic improvement. PLoS One. 2011. 6:e23366.

24. Lutz TA, Rand JS. Pathogenesis of feline diabetes mellitus. Vet Clin North Am Small Anim Pract. 1995. 25:527–552.

25. Mayr A. Infections which humans in the household transmit to dogs and cats. Zentralbl Bakteriol Mikrobiol Hyg B. 1989. 187:508–526.
26. Melo RCN, Fabrino DL, Dias FF, Parreira GG. Lipid bodies: structural markers of inflammatory macrophages in innate immunity. Inflamm Res. 2006. 55:342–348.

27. Mittelman SD, Van Citters GW, Kirkman EL, Bergman RN. Extreme insulin resistance of the central adipose depot in vivo. Diabetes. 2002. 51:755–761.

28. Panciera DL, Thomas CB, Eicker SW, Atkins CE. Epizootiologic patterns of diabetes mellitus in cats: 333 cases (1980-1986). J Am Vet Med Assoc. 1990. 197:1504–1508.
29. Portnoy DA, Jacks PS, Hinrichs DJ. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988. 167:1459–1471.

30. Pridal L, Deacon CF, Kirk O, Christensen JV, Carr RD, Holst JJ. Glucagon-like peptide-1(7-37) has a larger volume of distribution than glucagon-like peptide-1(7-36)amide in dogs and is degraded more quickly in vitro by dog plasma. Eur J Drug Metab Pharmacokinet. 1996. 21:51–59.

31. Rand JS, Fleeman LM, Farrow HA, Appleton DJ, Lederer R. Canine and feline diabetes mellitus: nature or nurture? J Nutr. 2004. 134:8 Suppl. 2072S–2080S.

32. Repp H, Pamukçi Z, Koschinski A, Domann E, Darji A, Birringer J, Brockmeier D, Chakraborty T, Dreyer F. Listeriolysin of Listeria monocytogenes forms Ca2+-permeable pores leading to intracellular Ca2+ oscillations. Cell Microbiol. 2002. 4:483–491.

33. Rotella CM, Pala L, Mannucci E. Glucagon-like peptide 1 (GLP-1) and metabolic diseases. J Endocrinol Invest. 2005. 28:746–758.

34. Schlech WF 3rd, Lavigne PM, Bortolussi RA, Allen AC, Haldane EV, Wort AJ, Hightower AW, Johnson SE, King SH, Nicholls ES, Broome CV. Epidemic listeriosis--evidence for transmission by food. N Engl J Med. 1983. 308:203–206.

35. Schoder D, Melzner D, Schmalwieser A, Zangana A, Winter P, Wagner M. Important vectors for Listeria monocytogenes transmission at farm dairies manufacturing fresh sheep and goat cheese from raw milk. J Food Prot. 2011. 74:919–924.

36. Skogberg K, Syrjänen J, Jahkola M, Renkonen OV, Paavonen J, Ahonen J, Kontiainen S, Ruutu P, Valtonen V. Clinical presentation and outcome of listeriosis in patients with and without immunosuppressive therapy. Clin Infect Dis. 1992. 14:815–821.

37. Slavov E, Georgiev IP, Dzhelebov P, Kanelov I, Andonova M, Georgieva TM, Dimitrova S. High-fat feeding and Staphylococcus intermedius infection impair beta cell function and insulin sensitivity in mongrel dogs. Vet Res Commun. 2010. 34:205–215.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download