Abstract
Human amniotic membrane-derived mesenchymal stem cells (hAM-MSCs) are capable of differentiating into several lineages and possess immunomodulatory properties. In this study, we investigated the soluble factor-mediated immunomodulatory effects of hAM-MSCs. Mitogen-induced peripheral blood mononuclear cell (PBMC) proliferation was suppressed by hAM-MSCs in a dose-dependent manner as well as hAM-MSC culture supernatant. Moreover, interferon-gamma and interleukin (IL)-17 production significantly decreased from PBMC, whereas IL-10 from PBMCs and transforming growth factor beta (TGF-β) production from hAM-MSCs significantly increased in co-cultures of hAM-MSCs and PBMCs. Production of several MSC factors, including hepatocyte growth factor (HGF), TGF-β, prostaglandin E2 (PGE2), and indoleamine 2, 3 dioxygenase (IDO), increased significantly in hAM-MSCs co-cultured with PBMCs. These results indicate that the immunomodulatory effects of hAM-MSCs may be associated with soluble factors (TGF-β, HGF, PGE2, and IDO), suggesting that hAM-MSCs may have potential clinical use in regenerative medicine.
Mesenchymal stem cells (MSCs) are multipotent non-hematopoietic progenitor cells which can differentiate into several mesenchymal lineages [4,10,18]. Another important characteristic of these cells is associated with their immunomodulatory properties. MSCs can suppress several types of immune cells including T [7,13], B [6], NK [23], and dendritic cells [29]. Furthermore, MSCs have been reported to ameliorate experimental murine autoimmune encephalomyelitis in a model of multiple sclerosis [28], prolong the survival of baboon skin in an allotransplation model [2], and control lethal graft vs. host complications [14]. These attributes have generated interest in the clinical use of MSCs in regenerative medicine [18].
Because bone marrow derived MSCs (BM-MSCs) represent a rare population (below 0.1% of nucleated cells) of adult cells, MSCs from alternative sources such as cord blood and adipose tissue are important. The placenta and its membranes, which are readily available and not associated with any substantial ethical issues, have received particular attention as a source of stem cells possessing multi- and pluripotent differentiation abilities. Furthermore, amniotic membrane-derived MSCs (AM-MSCs) inhibit allogeneic immune responses similar to BM-MSCs [4,15]. The major mechanism underlying immune-modulation by MSCs involves soluble factors such as transforming growth factor beta (TGF-β) [7], hepatocyte growth factor (HGF) [7], prostaglandin E2 (PGE2) [1] and indoleamine 2, 3 dioxygenase (IDO) [19]. In addition, cell-cell contact is also a possible factor that influences immune-modulation. However, its relevance to MSC function is not well understood.
In this study, we isolated human amniotic membrane-derived MSCs (hAM-MSCs) and investigated their characteristics and immunomodulatory effects. This was done to determine which factors are expressed and how expression of these factors is induced when hAM-MSCs are co-cultured with host immune cells. In this way, the possible use of hAM-MSCs for therapeutic use could be assessed.
hAM-MSCs in the third passage from different five donors were provided by RNL Bio, Korea. Briefly, human placentas were obtained after vaginal deliveries or caesarean section births from healthy women after obtaining informed consent. All human tissues were obtained with the approval of the Korea University Medical Center Institutional Review Board (Korea). The amnion were mechanically peeled from the placenta and washed with sterile saline several times to remove excess blood. Approximately 2.5 g of amnion tissues were cut into small pieces with scissors and digested with protease enzyme, collagenase type I (Gibco, USA), in shaking incubator at 37℃ for 1 h The digested tissues were filtered through 100 µm cell strainers (Falcon, USA) and centrifuged at 850 × g for 4 min. The pellet was resuspended in alpha-minimum essential medium (MEM; Gibco, USA) based medium containing 10% fetal bovine serum (FBS; PAA, Australia) and seeded into T75 flasks (Nunc, Denmark). The cultures were maintained at 37℃ in a humidified atmosphere with 5% CO2. Cell attachment was evaluated under a microscope 4 days after incubation and non-adherent cells were discarded by changing the medium. The cells were subcultured and expanded when the cells reached 90% confluence. The cells were used for the experiments at passage 3. The procedure for hAM-MSC preparation was performed under good manufacturing practice conditions [9].
The immunophenotype of the AM-MSCs was analyzed by flow cytometry (FACSCalibur; BD Biosciences, USA) using CellQuest software (BD Biosciences, USA). Antibodies against human antigens CD29 (BD555443), CD31 (BD555445), CD34 (BD555822), CD44 (BD555478), CD45 (BD555482), CD73 (BD550257), CD90 (BD555596), histocompatibility locus antigen (HLA)-ABC (BD555552), and HLA-DR (BD555811) were purchased from BD Pharmingen (USA). Antibody against human antigen CD105 (FAB10971P) was purchased from R&D Systems (USA). Trypsinized cell were suspended in 5% bovine serum albumin and stained with specific antibody for 2 h. After stainining, cells were analyzed flow cytometry.
At 50% confluence, hAM-MSCs were cultured for 14 days in NH Osteodiff Medium (Miltenyi Biotec, Germany) with 90% of the medium replaced every 3 days. Cells were stained with Alizarin red S (Sigma-Aldrich, USA). Cells were fixed in 70% ethanol, stained with 40 mM Alizarin Red S solution (pH 4.2) for 1 h. After staining, cells were washed with PBS and observed a microscope (Axiovert 300; Carl Zeiss, Germany).
At 80% confluence, AM-MSCs were cultured for 21 days in adipogenic induction medium contained low glucose-Dulbecco's modified Eagle medium (DMEM) with 10% FBS, 200 µM indomethacin (Sigma-Aldrich, USA), 1 µg/mL insulin (Sigma-Aldrich, USA), 1 mM dexamethasone (Sigma-Aldrich, USA), 0.5 mM isobutylmethylxanthine (Sigma-Aldrich, USA), 100 U/mL penicillin (Gibco, USA), 100 mg/mL streptomycin (Gibco, USA), and 0.25 mg/mL amphotericin B (Gibco, USA). The medium was replaced with adipocyte induction medium or control (stromal) medium, alpha-MEM (Gibco, USA)-based medium containing 5% FBS (PAA, Australia) with every 3 days. After cells were differentiated into adipocytes completely for 2 weeks, cells were fixed in 10% formalin and then stained with oil red O solution [40% H2O and 60% oil red O stock solution composed of 0.5% oil red O (Sigma-Aldrich, USA) in isopropanol (Sigma-Aldrich, USA)] for 1 h. After staining, cells were washed with distilled water and observed by a microscope (Axiovert 300; Carl Zeiss, Germany).
hAM-MSCs (5 × 105 cells) cultured in alpha-MEM (Gibco, USA)-based medium containing 5% FBS (PAA, Australia) were centrifuged at 500 × g for 5 min. The cells were then resuspended in 0.5 mL of commercialized NH chondrogenic medium (Miltenyi Biotec, Germany) containing dexamethasone, ascorbate, insulin-transferrin-selenium, penicillin, sodium pyruvate, praline, L-glutamine and TGF-β. The cells were centrifuged again at 500 × g for 5 min to form pellets. The pellets were cultured in polypropylene tubes for 14 days with 50% of the chondrogenic induction medium being replaced every 3 to 4 days. After cells were differentiated into chondrocytes completely for 3 weeks, cellswere fixed in 10% formalin, stained with 1% toluidine blue for 15 min and then washed with distilled water and observed by a microscope (Axiovert 300; Carl Zeiss, Germany).
Peripheral blood mononuclear cells (PBMCs) were obtained from healthy volunteers. Briefly, peripheral blood sample was diluted with an equal volume of phosphate buffered saline, layered onto a Histopaque (specific gravity 1.077; Sigma-Aldrich, USA) and centrifuged at 600 × g for 20 min. PBMCs were collected from the interphase and washed twice with PBS. Cell viability was analyzed by trypan blue exclusion.
hAM-MSCs (1 × 103 ~ 5 × 104) irradiated with Cs137 (3,000 rad) using GC 3000 Elan (MDS Nordion, Canada) were seeded onto 96-well flat bottom plates (Costar, USA). PBMCs (1 × 105/100 µL) were added to the layer of hAM-MSCs and then cultured with concanavalin A (ConA, 5 µg/mL; Sigma-Aldrich, USA) for 72 h. For some experiments, PBMCs (1 × 105 cells/100 µL) were seeded in triplicate in 96-well round-bottom plates (Costar, USA) in the presence of supernatant obtained from hAM-MSCs (1 × 105 cells) cultured in 6-well plates (Costar, USA) for 72 h. Leukocyte proliferation was evaluated using a cell proliferation BrdU ELISA kit (Roche Diagnostics, Germany) according to the manufacturer's instructions. The stimulation index value (mean optical density values of mitogen-stimulated cultures/mean optical density values of no mitogen-stimulated cultures) was calculated for each treatment.
hAM-MSCs (1 × 105 cells) were seeded in 24-well plates (Costar, USA). PBMCs (1 × 106 cells) were added onto the MSC layer and cultured with ConA (5 µg/mL) for 72 h. PBMCs cultured without hAM-MSCs served as a control. To determine the amount of interferon-gamma (IFN-γ), interleukin (IL)-17, TGF-β, IL-10, and PGE2 in the cell-free culture supernatant, commercial ELISA kits (Cayman Chemicals, USA) were used according to the manufacturer's instructions.
To determine the amount of IDO enzymatic activity catalyzing the degradation of the tryptophan to kynurenine, the level of its metabolite, kynurenine, was measured spectrophotometrically. Briefly, 50 µL of 30% trichloroacetic acid (Sigma-Aldrich, USA) was added to 100 µL of culture supernatant (hAM-MSCs only, co-culture of hAM-MSCs and PBMCs and co-culture of hAM-MSCs and PBMCs and ConA; 5 µg/mL), incubated at 50℃ for 30 min, and then centrifuged at 15,400 × g for 1 min. The supernatant (75 µL) was then added to an equal volume of Ehrlich's reagent (100 mg p-dimethylbenzaldehyde and 5 mL glacial acetic acid; Sigma-Aldrich, USA) in a 96-well plate. Optical density was measured at 490 nm using microplate reader 680 (Bio-Rad, USA).
After 12 h, PBMCs were removed from hAM-MSC/PBMC co-cultures by gentle PBS washing and total RNA was then extracted using Easy-Blue (Intron, Korea) according to the manufacturer's instructions. In some experiments, hAM-MSCs were separated from PBMCs by a Transwell membrane (0.3 µm pore size; Costar, USA). Reverse transcription of 2 µg of total mRNA was conducted using a cDNA synthesis kit (Takara, Japan) according to manufacturer's recommendations. PCR amplification of cDNA aliquots was then performed in a Mastercycler (Eppendorf, Germany) by adding 2.5 mM dNTPs, 2.5 U Taq DNA polymerase (Intron, Korea) and 0.25 µM forward and reverse primers through 30 cycels of 94℃ for 20 sec, 55℃ for 20 sec, and 72℃ for GAPDH and 32 cycels of 94℃ for 20 sec, 55℃ for 20 sec, and 72℃ for 30 sec for TGF-β, HGF, IDO and cyclooxygenase-2 (COX-2). Primer sequences were as follows: GAPDH (forward: 5'-aacatcatccctgcttccac-3', reverse: 5'-gaccacctggtcctcagtgt-3'), TGF-β (forward: 5'-agcaccctacttgaggcga-3', reverse: 5'-actggatcatctccgacagg-3'), HGF (forward: 5'-atcacccagaatcaggcatc-3', reverse: 5'-tttccatggcgataaactcc-3'), IDO (forward: 5'-ctcagattactacaaccgatc-3', reverse: 5'-accaggatctcttgctggat-3'), and COX-2 (forward: 5'-atgctgctaacctaata-3', reverse: 5'-ctagtgaggctatctttat-3').
The expression of hAM-MSC surface markers was determined by flow cytometry. Five samples of hAM-MSCs were uniformly positive for CD29 (98.4 ± 1.0%), CD44 (99.0 ± 0.7%) CD73 (97.4 ± 18.3%), CD90 (99.8 ± 0.1%), and CD105 (70.3 ± 13.3%), but negative for CD31 (0.4 ± 0.6%), CD34 (0.4 ± 0.5%), and CD45 (0.3 ± 0.6%). Immunologically, the hAM-MSCs were positive for HLA-ABC but negative for HLA-DR (Fig. 1A). Next, we investigated the gene expression profile of the hAM-MSCs by RT-PCR. As shown in Fig. 1B, the hAM-MSCs constitutively expressed COX-2, HGF, and TGF-β mRNA; however, they did not constitutively express IDO and IL-10 mRNA.
The hAM-MSCs underwent differentiation when induced with specific factors (Fig. 1C). Osteogenic differentiation was confirmed by positive Alizarin red S staining. Almost all cells were found to undergo osteogenic differentiation shown by red mineralized deposits. Adipogenic differentiation was assessed by positive oil red O staining and accumulation of lipid vacuoles within the cells. Chondrogenic differentiation was associated with round-shaped inclusions located in lacuna-like structures within the cell detected by toluidine blue O staining.
To determine whether hAM-MSCs could suppress mitogen-induced proliferation of allogeneic lymphocytes, PBMCs were stimulated with ConA in the presence of hAM-MSCs for 72 h. Allogeneic PBMC proliferation as a result of mitogen treatment was inhibited by AM-MSCs (Fig. 2; p < 0.05). To evaluate whether the number of hAM-MSCs was related to the degree of suppression of PBMC proliferation, various amounts of hAM-MSCs were co-cultured with PBMCs. A shown in Fig. 2A, hAM-MSCs inhibited ConA-induced PBMC proliferation in a dose-dependent manner. When hAM-MSCs were incubated with PBMCs at ratios of 1 : 2 and 1 : 10, PBMC proliferation was successfully abrogated (p < 0.05). However, there was no significant difference in proliferation when hAM-MSCs were incubated with PBMCs at a ratio of 1 : 100 compared to that observed without the addition of hAM-MSCs (p > 0.05). Supernatant from hAM-MSC cultures was added to PBMC cultures stimulated with ConA in order to determine whether soluble factors secreted by hAM-MSCs were associated with the immunomodulatory effect we observed. Leukocyte proliferation in response to mitogenic stimulus was blocked by the addition of hAM-MSC culture supernatant (Fig. 2B).
To examine the effect of hAM-MSCs on the activity of the immune cells, we measured the production of IL-17, IFN-γ, TGF-β, and IL-10 in culture supernatant obtained from a co-culture of hAM-MSCs (1 × 105) and PBMCs (1 × 106). As shown in Fig. 3, lower levels of IL-17 and IFN-γ production were observed in the supernatant from co-cultures of hAM-MSCs and PBMCs in the presence of mitogens compared to the supernatant obtained from cultures of PBMCs alone (p < 0.05). However, the level of IL-10 and TGF-β production increased significantly in the supernatant obtained from the co-cultures of hAM-MSCs and PBMCs (p < 0.01).
Among all molecules we evaluated, mRNA expression of TGF-β, HGF, IDO, and COX-2 was induced more, not only in hAM-MSCs grown in the presence of PBMCs but also in hAM-MSCs separated from PBMCs by transwells compared to those grown without PBMCs (Fig. 4A). PGE2 and IDO are known key factors of immune tolerance as well as immune modulators of MSCs. Therefore, we measured PGE2 and kynurenine levels in the supernatant obtained from hAM-MSC/PBMC co-cultures. As shown in Figs. 4B and C, higher levels of kynurenine production and PEG2 were observed in the supernatant obtained from the co-culture.
MSCs have received attention for their differentiation ability and therapeutic potential in regenerative medicine. Although the mechanism underlying the immune-modulation of MSCs remains to be elucidated, it is now clear that these cells can suppress allogeneic immune responses. Several studies have recently shown that amniotic membrane-derived cells display immunomodulatory properties similar to those described for MSCs obtained from other sources such as bone marrow and adipose tissue [4,15]. Similar to other MSCs, the mechanism of immune-modulation by hAM-MSCs is yet to be clarified. In the present study, we demonstrated the following: (1) hAM-MSCs inhibited mitogen-induced proliferation of allogeneic PBMCs; (2) these effects were caused by not only contact with hAM-MSCs but also MSC culture supernatant; (3) hAM-MSCs caused PBMCs to secrete anti-inflammatory cytokines, especially IL-10; and (4) hAM-MSCs produced IDO, PGE2, TGF-β, and HGF, factors that might induce suppression of proliferation and inflammatory cytokines production of immune cells. These data indicate that those suppressive effects on proliferation and inflammatory cytokines production of immune cells by hAM-MSCs might be partially mediated by soluble factors, which is consistent with findings from other types of MSCs [1,13].
IL-10 is a well-known cytokine involved in cell regulation and promotion of proliferation and activation of regulatory or anti-inflammatory cells. Previous studies have been performed that show increases in IL-10 production in a mixed lymphocyte population co-cultured with hBM-MSCs [1,3,11,12] and in plasmacytoid dendritic cells (DCs) co-cultured with BM-MSCs and human adipose tissue-derived MSCs (hAD-MSCs) [27]. On the other hand, other groups found no change in IL-10 production when hBM-MSCs and PBMCs were co-cultured and stimulated with phytohaemagglutinin [21], or IL-10 did not detected when hAD-MSCs and PBMCs were co-cultured [20]. Our data showed increased levels of IL-10 in supernatant from co-cultures of hAM-MSCs and PBMCs. hAM-MSCs did not produce IL-10. Rather, IL-10 was secreted from PBMCs, indicating that T cells or antigen presenting cells might be influenced by a paracrine effect. Soluble factors from mouse BM-MSCs induce IL-10 production by mouse splenocytes, which in turn suppress the proliferation of T cells by inducing apoptosis [25]. Furthermore, production of HLA-G, a known immunomodulatory factor, is induced via IL-10 [17]. However, there are no previous studies that investigated induction of IL-10 production by hAM-MSCs. Thus, further investigations are needed to verify the effects of IL-10 induced by hAM-MSCs on immune cells and the mechanism of induced IL-10 production by hAM-MSCs.
According to previous reports, anti-TGF-β antibody abrogates immune suppression by MSCs and recombinant TGF-β enhances the immunomodulatory properties of MSCs [7,23]. However, Le Blanc et al. [13] and Tse et al. [25] reported no significant restoration in T-cell proliferation as a result of treatment with anti-TGF-β antibody. We observed increased expression of TGF-β mRNA in hAM-MSCs 12 h after co-culturing with leukocytes and the constitutive expression of TGF-β mRNA in hAM-MSCs. Furthermore, we observed an increased level of TGF-β in the culture supernatant obtained from hAM-MSCs and PBMCs co-cultured for 3 days, indicating that TGF-β secreted by hAM-MSCs may participate in the immunomodulatory effect on PBMCs.
IDO, a well-known immune-suppression factor not expressed constitutively in MSCs, inhibits T-cell proliferation and promotes immune tolerance by tryptophan depletion [16]. Although Tse et al. [25] reported that IDO inhibitors do not restore T-cell proliferation, other studies showed that IDO production in hBM-MSCs are induced by IFN-γ produced by T cells. Moreover, hBM-MSCs induce T-cell apoptosis via IDO [18] and T-cell proliferation is restored by IDO inhibitors [12]. In our study, IDO mRNA was not constitutively expressed in hAM-MSCs. However, IDO mRNA and kynurenine production increased when hAM-MSCs and PBMCs were co-cultured, suggesting that IDO was induced by co-culturing and participated in immune modulation by hAM-MSCs.
PGE2, one of the MSC immune-modulation candidates, that is synthesized from arachidonic acid by COX-1 and COX-2 enzymes regulates the maturation and antigen presentation of DC, and inhibits T cell proliferation and cytokine production [26]. MSCs constitutively express the COX enzymes, indicating that PGE2 is also constitutively expressed. According to Aggarwal et al. [1], the PGE production pattern is bell-shaped and occurs in a time-dependent manner, indicating that this factor might be related to early immune suppression by MSCs. Our results also showed that COX-2 mRNA and PEG2 production increased in hAM-MSCs when they were co-cultured with PBMCs. A previous study showed that PGE2 and TGF-β from BM-MSCs induce the proliferation of CD4+ CD25high FOXP3+ regulatory T cells [8].These induced Treg cells suppressed allogeneic immune responses, indicating that soluble factors from MSCs do not solely suppress immune responses [8]. Moreover, blocking PGE2 production in the T cells/hAD-MSCs co-culture resulted in a significant decrease of IL-10 production [26]. PGE2 is the most powerful immunomodulatory factor in human umbilical cord-derived MSCs (hUC-MSCs). This is because inhibition of PGE synthesis almost completely mitigates the immunosuppressive effects whereas neutralization of TGF-β and IDO has little effect [5]. Thus, further studies are necessary to determine whether hAM-MSCs induce the proliferation of Treg cells via PGE2 and TGF-β.
Supernatants from hAM-MSCs had a partial inhibitory effect on PBMC proliferation suggesting that presence of soluble factors in the supernatant might have inhibited PBMCs proliferation. Thus, further studies on the sufficient induction of soluble factors from hAM-MSCs by pretreatment with inducers or genetic engineering may be needed before these hAM-MSCs can be used for clinical purposes. Although we found that soluble factors inhibited the proliferation and inflammatory cytokines production of immune cells, cell-cell contact between MSCs and lymphocytes is also one of the possible mechanisms underlying the immuno-modulatory effects of MSCs. Cell-cell contact between BM-MSCs and lymphocytes induced IL-10 and TGF-β transcription in BM-MSCs. Moreover, cell adhesion molecules, such as B7-H1, intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), may participate in immune-modulation. Immune-modulation by hUC-MSCs is largely mediated by cell-cell contact via adhesion molecules, particularly B7-H1 [24]. Moreover, the adhesion molecules ICAM-1 and VCAM-1 mediated immunosuppression in mouse BM-MSCs induced by IFN-γ from activated T cells; this activity was abrogated by antibodies against ICAM-1 and VCAM-1 [22]. Thus, cell-cell contact between hAD-MSCs and PBMCs may play an important role in the immunomodulatory effects of hAD-MSCs. Further studies examining the effects of cell-cell contact on the immunomodulatory properties of hAD-MSCs are necessary.
There are some differences in immune-modulation mechanisms among human MSCs from various sources. These differences could be explained by methodological differences such as the co-culture time, ratio of MSCs and immune cell in the co-culture, or source of the MSCs. Therefore, further studies should be performed to measure the effect of these differences on the immune-modulation effects of MSCs and establish a standardization system for these various methods. In conclusion, we found that soluble factors produced by hAM-MSCs might suppress allogeneic immune responses via induction of IL-10 production, supporting the hypothesis that these cells have potential therapeutic use. Further study is needed to identify the detailed mechanisms responsible for the immunomodulatory effects of hAM-MSCs.
Figures and Tables
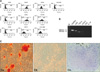 | Fig. 1Characterization of human amniotic membrane-derived mesenchymal stem cells (hAM-MSCs). (A) Immunophenotyping of hAM-MSCs at passage 3 by fluorescence activated cell sorting analysis. Cells were positive for CD29, CD44, CD73, CD90, CD105, and HLA-ABC but negative for CD31, CD34, CD45, and HLA-DR. (B) Constitutive mRNA expression of angiogenic growth factor (hepatocyte growth factor [HGF]), cytokines (transforming growth factor beta [TGF-β] and interleukin [IL]-10]), cyclooxygenase-2 (COX-2), and indoleamine 2, 3 dioxygenase (IDO) in steady state hAM-MSCs measured by RT-PCR. GAPDH mRNA was used as an internal control. M: 100-bp molecular weight ladder. (C) Differentiation of hAM-MSCs. (Ca) Osteogenic differentiation analyzed by Alizarin red S staining, (Cb) adipogenic differenction by oil red O staining, and (Cc) chondrogenic differentiation assessed by toluidine blue O staining. ×100. |
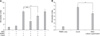 | Fig. 2Suppressive effects of hAM-MSCs on the proliferation of peripheral blood mononuclear cell (PBMC): (A) PBMCs (1 × 105 cells) cultured with ConA (5 µg/mL) in the presence or absence of irradiated hAM-MSCs (1 × 103 ~ 5 × 104 cells) for 3 days. (B) PBMCs (1 × 105 cells/100 µL) cultured with supernatant obtained from hAM-MSCs (100 µL) cultured for 3 days. Data are expressed as the mean ± SE of four independent experiments. *p < 0.05, **p < 0.01. |
 | Fig. 3Cytokine levels in supernatant of co-cultured hAM-MSCs and PBMCs stimulated with mitogen. PBMCs (1 × 106 cells) cultured with ConA (5 µg/mL) in the presence or absence of hAM-MSCs (1 × 105 cells) for 3 days. Production of TGF-β (A), IL-10 (B), IL-17 (C), and interferon-gamma (IFN-γ; D) was assessed in 3-day culture supernatant using a sandwich ELISA kit. Data are expressed as the mean ± SE of five independent experiments. ND: not detected, *p < 0.05, **p < 0.01. |
 | Fig. 4Induction of immunomodulatory factor production in hAM-MSCs by co-culturing with PBMCs. hAM-MSCs (1 × 105 cells) cultured with or without PBMCs (1 × 106 cells) in the presence of ConA (5 µg/mL) for 12 h or 3 days. (A) mRNA expression of COX-2, TGF-β, HGF, and IDO in AM-MSCs cultured with or without PBMCs for 12 h was evaluated by RT-PCR. GAPDH was used as an internal control. (B) IDO enzymatic activity was indirectly determined spectrophotometrically by measuring the amount of kynurenine in 3-day culture supernatant from hAM-MSCs/PBMCs co-cultures. (C) The level of prostaglandin E2 in the supernatant from co-cultured hAM-MSCs and PBMCs was determined by sandwich ELISA. **p < 0.01. |
Acknowledgments
We are grateful for Ms. Sook Shin, Dr. Il Seob Shin, and Mr. In Su Choi for their outstanding technical assistance. This study was supported by RNL Bio Research Grant. Additional support was provided by the Seoul R&BD program (No.10548), the Korean Research Foundation Grant (KRF-2006-005-J02903), Research Institute of Veterinary Science, Department of Veterinary Microbiology, College of Veterinary Medicine, and the BK21 Program for Veterinary Science, Seoul National University, Korea.
References
1. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005. 105:1815–1822.

2. Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002. 30:42–48.

3. Batten P, Sarathchandra P, Antoniw JW, Tay SS, Lowdell MW, Taylor PM, Yacoub MH. Human mesenchymal stem cells induce T cell anergy and downregulate T cell allo-responses via the TH2 pathway: relevance to tissue engineering human heart valves. Tissue Eng. 2006. 12:2263–2273.

4. Chang CJ, Yen ML, Chen YC, Chien CC, Huang HI, Bai CH, Yen BL. Placenta-derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon-gamma. Stem Cells. 2006. 24:2466–2477.

5. Chen K, Wang D, Du WT, Han ZB, Ren H, Chi Y, Yang SG, Zhu D, Bayard F, Han ZC. Human umbilical cord mesenchymal stem cells hUC-MSCs exert immunosuppressive activities through a PGE2-dependent mechanism. Clin Immunol. 2010. 135:448–458.

6. Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006. 107:367–372.

7. Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002. 99:3838–3843.

8. English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. Cell contact, prostaglandin E2 and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+ CD25High forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009. 156:149–160.

9. Hwang JH, Shim SS, Seok OS, Lee HY, Woo SK, Kim BH, Song HR, Lee JK, Park YK. Comparison of cytokine expression in mesenchymal stem cells from human placenta, cord blood, and bone marrow. J Korean Med Sci. 2009. 24:547–554.

10. Kang JW, Kang KS, Koo HC, Park JR, Choi EW, Park YH. Soluble factors-mediated immunomodulatory effects of canine adipose tissue-derived mesenchymal stem cells. Stem Cells Dev. 2008. 17:681–693.

11. Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW, Deans RJ, McIntosh KR. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005. 12:47–57.

12. Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, Romagnani P, Maggi E, Romagnani S, Annunziato F. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006. 24:386–398.

13. Le Blanc K, Rasmusson I, Götherström C, Seidel C, Sundberg B, Sundin M, Rosendahl K, Tammik C, Ringdén O. Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol. 2004. 60:307–315.

14. Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004. 363:1439–1441.

15. Li C, Zhang W, Jiang X, Mao N. Human-placenta-derived mesenchymal stem cells inhibit proliferation and function of allogeneic immune cells. Cell Tissue Res. 2007. 330:437–446.

16. Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004. 4:762–774.

17. Nasef A, Chapel A, Mazurier C, Bouchet S, Lopez M, Mathieu N, Sensebé L, Zhang Y, Gorin NC, Thierry D, Fouillard L. Identification of IL-10 and TGF-β transcripts involved in the inhibition of T-lymphocyte proliferation during cell contact with human mesenchymal stem cells. Gene Expr. 2007. 13:217–226.

18. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.

19. Plumas J, Chaperot L, Richard MJ, Molens JP, Bensa JC, Favrot MC. Mesenchymal stem cells induce apoptosis of activated T cells. Leukemia. 2005. 19:1597–1604.

20. Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, Taureau C, Cousin B, Abbal M, Laharrague P, Penicaud L, Casteilla L, Blancher A. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005. 129:118–129.

21. Rasmusson I, Ringdén O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005. 305:33–41.

22. Ren G, Zhao X, Zhang L, Zhang J, L'Huillier A, Ling W, Roberts AI, Le AD, Shi S, Shao C, Shi Y. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol. 2010. 184:2321–2328.

23. Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006. 24:74–85.

24. Tipnis S, Viswanathan C, Majumdar AS. Immunosuppressive properties of human umbilical cord-derived mesenchymal stem cells: role of B7-H1 and IDO. Immunol Cell Biol. 2010. 88:795–806.

25. Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003. 75:389–397.

26. Yañez R, Oviedo A, Aldea M, Bueren JA, Lamana ML. Prostaglandin E2 plays a key role in the immunosuppressive properties of adipose and bone marrow tissue-derived mesenchymal stromal cells. Exp Cell Res. 2010. 316:3109–3123.

27. Yang SH, Park MJ, Yoon IH, Kim SY, Hong SH, Shin JY, Nam HY, Kim YH, Kim B, Park CG. Soluble mediators from mesenchymal stem cells suppress T cell proliferation by inducing IL-10. Exp Mol Med. 2009. 41:315–324.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download