Pylorogastric intussusception (PGI) is a retrograde intussusception, which is an uncommon condition [2,3,5-7]. Ultrasonographic examination is a fairly useful modality for the diagnosis of intussusception with high sensitivity and specificity, however only a maltese dog with a PGI diagnosed by ultrasonography was described [6,8,9].
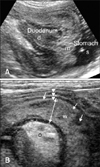
A 15 year-old, spayed female, Yorkshire terrier (case 1) presented with acute, severe vomiting and hematemesis for 1 day. Blood tests revealed neutrophilia, elevated amylase and decreased potassium and chloride. In transverse ultrasonographic view, a target-like mass (38 × 31 mm) with multiple concentric rings occupied the body of the stomach (Fig. 1). Multiple hyperechoic and hypoechoic parallel lines in longitudinal view were shown and connected with the descending duodenum. The stomach was dilated with anechoic fluid. The mass represented the invagination of the pylorus and the proximal duodenum into the body of the stomach. The pyloric wall was swollen to 7.1 mm thickness and showed indistinct wall layering with multiple hypoechoic regions. The adjacent mesentery was edematous with hyperechoic change. The pancreas was not involved in invagination. A PGI and pyloric wall necrosis were diagnosed. On laparotomy, the pylorus and the proximal duodenum were invaginated into the body of the stomach (Fig. 2). The pyloric wall was extremely congested and necrotic, and manual reduction was impossible, even after a gastrotomy incision was made in the body of the stomach. The necrotic pyloric wall and the invaginated pylorus were removed through a partial gastrectomy, leaving only about 25% of the gastric pylorus and then a Y-U pyloroplasty was adopted. The extensive hemorrhage and necrosis in muscular layer of the removed pylorus were found in histopathologic examination. After surgery, the dog was recovered from anesthesia uneventfully and vomiting disappeared.
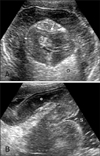
A 9 year-old, female, Miniature Schnauzer (case 2) presented with chronic, intermittent vomiting, abdominal pain and depression for 6 days. Blood tests revealed decreased potassium, sodium and chloride. Survey abdominal radiography revealed that a mass with soft tissue opacity was located in the stomach and the pyloric lumen was filled with gas. Ultrasonography showed a mass consisted of concentric multiple, hyperechoic and hypoechoic rings in the body of the stomach, which was identified as the invaginated pylorus into the gastric body, and it was connected with the descending duodenum (Fig. 3). The pyloric wall was swollen to 13 mm thickness and had homogeneous, distinguishable wall layers. The pancreas was not involved in invagination. A PGI was diagnosed. During conservative therapy, vomiting decreased and a PGI was reduced spontaneously ultrasonographically.
PGIs can result in necrosis of gastric wall in cases with blood vessels compromised in intussusceptions or with chronic condition and eventually cause poor prognosis [3,7]. A prompt diagnosis and treatment would be mandatory. However, a previous maltese dog of PGI had chronic progress of clinical signs and resolved spontaneously [6]. In this study, a PGI in case 1 was required prompt surgical correction because of gastric congestion and necrosis, but a PGI in case 2 resolved spontaneously. It is difficult to estimate even if there are the predictive ultrasonographic factors to the suitable treatments and prognosis of PGIs, because PGIs in most previous cases which underwent surgery were diagnosed through only radiography and exploratory laparotomy [2,3,5,7]. Radiography in PGIs may demonstrate the gastric outflow obstruction with soft tissue density masses, which can be shown in gastric foreign body, pyloric hyperplasia and stenosis, or gastric neoplasia [2,3,5,7]. The previous maltese dog diagnosed by ultrasonography and our case 2 showed homogeneous hypoechoic mucosal and muscular layers with even thickness and distinct wall layering in the invaginated gastric wall. In case 1, the every layers of the invaginated pyloric wall were thickened unevenly and indistinguishable. Multiple hypoechoic lesions were observed in the pyloric wall, which represented severe edema and diffuse necrosis of the gastric wall, as confirmed during laparotomy. The gastric necrosis may be an evidence of vascular compromise, although the vascular supply adjacent to the mesentery around the PGI was not evaluated in our cases. Evaluation of the vascular supply to the associated tissue is critical to evaluate the risk of subsequent gastric necrosis or perforation, and the acuity and severity of clinical signs in intussusceptions are directly associated vascular compromise [1]. The invagination of the pancreas into the intussusceptions was assumed as an exacerbating factor for severe necrosis of the gastric wall in a previous report [3]. In the maltese case and our cases, the pancreas was not involved the PGIs, so we couldn't estimate the relationship between the invagination of pancreas and the progress of the PGIs. In addition to these preoperative factors, we speculated that the surgical method would influence on the prognosis of dogs with PGIs. Among previous 4 cases underwent the laparotomies, one dog was euthanized because the necrotic gastric wall could not reduced and another dog was died by sepsis after surgery. Despite our case 1 had profound necrosis of the gastric wall, the resection of the extensive necrotic wall could reduce the possibility of sepsis after surgery and a Y-U pyloroplasty supported the dog's recovery by increasing the diameter of the pyloric outflow tract [4].
Because only a few cases of PGI have been reported in veterinary medicine, it is difficult to speculate on the etiology of PGIs. Previous surgery and anesthesia, and acute gastroenteritis have been suggested as predisposing factors of PGIs [9]. Case 1 had a history of intestinal resection and anastomosis due to an intestinal foreign body and perforation and case 2 underwent nephrectomy for pyonephrosis and peritonitis before PGI developed.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download