Abstract
Recently, we reported the three wolves cloning with normal karyotype from somatic cells of endangered male gray wolves (Canis lupus), but one wolf had female external genitalia. In this study, we conducted further clinical, histological, and genetic analyses. This cloned wolf had a normal uterus but developed ovotestis. Through molecular analysis of the SRY gene, a mutation in the coding sequence of SRY gene could be excluded as a cause of intersexuality. This is the first report of a cloned wolf with a 78, XY ovotesticular disorder affecting sexual development characterized by bilateral ovotestes.
Birth of the first canine puppy derived from somatic cell nuclear transfer was reported in 2005 [5]. We recently reported the successful cloning of endangered gray wolves (Canis lupus) from somatic cells collected postmortem [7]. In this study, three live cloned wolves were delivered. All of the pups had a normal male wolf karyotype (78, XY) but one had female external genitalia. While male pseudohermaphroditism has been described in numerous species [9], true hermaphroditism in a wolf has never been reported. Here, we describe a case of intersexuality in a cloned wolf that was evaluated using clinical, histological, and genetic methods.
A 1.5-year-old gray wolf (Canis lupus) cloned from male somatic cells collected postmortem developed female external genitalia including a small vulva instead of testicles and a penis. On physical examination, the wolf was found to have a normal sized vulva and small clitoris in the normal anatomical positions. A reddened, smooth mucosa was observed with vaginoscopy and the urethral orifice was located cranially to the base of the clitoris. Vaginal cytology showed predominantly intermediate and parabasal cells indicative of anestrus. Complete blood count results and serum biochemistry profiles were within normal canine reference ranges. Radiologic examination (reproductive tract radiography) with 300 mg/mL of Omnipaque (Iohexol; Armersham health, Ireland) as a contrast medium was performed to identify structures of the vagina, uterine body, uterine horn, and cervix. Abdominal ultrasonographic scanning with a 7.0 MHz transducer revealed ovary-like structures located caudal to the kidney; the right one was 1.58 × 0.98 cm and the left was 1.55 × 0.95 cm.
In order to measure the plasma levels of testosterone [1], gonadotropin-releasing hormone (GnRH) was first administered intravenously (100 µg/mL of Gonadorelin [Gonadon; Dongbang, Korea], 20 IU/kg body weight). Sequential blood samples were then taken at 0, 30, 60, and 90 min. Results of the GnRH stimulation test were indicative of gonadal failure or an absence of male gonads. Baseline testosterone levels were <1 ng/mL with little response to GnRH. Also, Cytogenetic techniques revealed that the affected wolf had a normal male genotype (78, XY). The size and morphology of both the X and Y chromosomes were also normal (Fig. 1C).
Exploratory laparotomy was performed in order to observe the reproductive tract more clearly. The animal was first given atropine sulfate (0.1 mg/kg, Atropin; Jeil Pharm., Korea) subcutaneously, and then cephradine (22 mg/kg, Tri-cepa; Kukje Pharm., Korea), tramadol (4 mg/kg, Dorazzin; Samsung Pharm., Korea) intravenously. Anesthesia was then induced with propofol (6 mg/kg, Provive 1%; Claris Lifesciences, India) administered intravenously and maintained with 2% isoflurane (Ifran; Hana Parm., Korea) in oxygen. The ventral abdomen was shaved and prepared for aseptic surgery. A midline laparotomy was performed from the umbilicus to the pubic bone. The entire abdomen was thoroughly evaluated for congenital abnormalities. The internal genitalia organs were removed by routine surgical procedures. We observed a macroscopically normal uterus and bilateral ovary-like structures located at the end of the uterine horns (Fig. 1A). The surgically excised tissues were taken for routine hematoxylin and eosin staining and light microscopy. Histologically, the uterus appeared to be normal and the ovary-like masses were lined with a germinal surface epithelium. Scattered clusters of primitive follicle-like structures, comparable to those in immature ovaries, were present in the cortex of mass. Multiple tubular structures with a prominent basement membrane were observed in the medulla. The tubules were lumenized and composed of large cells with pale cytoplasm and basal round nuclei. The gonadal medullary tubular structures were histologically identical to immature seminiferous tubules with immature Sertoli cells. No germ cells were observed. Since both ovarian and testicular components were present, the masses were classified as ovotestes (Fig. 1B).
To screen for mutations in the SRY gene, we analyzed DNA samples from the affected wolf. Genomic DNA was extracted from skin fibroblasts cultures using DNA extraction kit (iNtRON Biotechnology, Korea) according to the manufacturer's instructions. Internal SRY gene amplification was performed by PCR using the appropriate primers: Canine SRY primer sequence was a positive strand 5'cccggtatgttgacctt-3'and a negative strand 5'ggcctgta gtctctgcgcctc-3'(SRY1), 5'agcagctggggtaccagtgga-3'and a negative strand 5'gccataaacccagcctgagtc-3'(SRY2), and 5'actctggctaacgaaacgtcct-3 and a negative strand 5'aaaga gtgaaggtccacccgg-3'(SRY3). Amplified fragments of the SRY gene were subjected to DNA sequencing analysis with an ABI3100 instrument (Applied Biosystems, USA). When comparing samples derived from the affected animal, its litter-mates, and the somatic cell donor, we did not find any differences in the full sequence of the SRY gene (1,578 bp, data not shown).
Sex-reversal has been reported in mammals among numerous species of domestic animals; this has been observed only few times in wild animals [8,10]. Interestingly, in the cloned wolf derived from male postmortem tissues we examined in this study, female external genitalia but no testes and penis were observed. The affected wolf was classified as an XY true hermaphrodite due to the presence of bilateral ovotestes containing seminiferous tubules with ovarian tissues, internal (uterus) and external (clitoris and vulva) female genitalia, and the SRY gene in the genomic DNA sequence. XY sex-reversal results in the presence of ovarian tissue in addition to testicular tissue (XY true hermaphrodites) [4]. However, the precise classification of sex development disorders is unclear, and attempts to develop appropriate nomenclature are ongoing. An interesting proposal was recently presented by Poth et al. [9]. According to their specifications, the wolf in our study should be classified as having a 78, XY ovotesticular disorder of sex development (DSD).
In mammals, normal male sex determination is mediated by the SRY gene located on the Y chromosome [3]. Mutations in the SRY gene cause failure of testicular development that generally results in phenotypically normal females with gonadal dysgenesis and complete male-to-female sex-reversal [6]. Because the gonads in such affected individuals are generally dysgenetic and nonfunctional, spontaneous pubertal development may not occur as observed in our case. Therefore, we analyzed the sex-determining region of the Y chromosome, including the SRY gene, to look for mutations in the affected wolf. No mutations in the SRY sequence were detected. Thus, a mutation in the coding sequence of the SRY gene can be excluded as a cause of intersexuality in our case. Although SRY encodes a testis-determining factor that promotes male sexual development, induction of testis development can occur in the absence of SRY as observed in domestic animals [2] with SRY-negative XX sex-reversal. Therefore, further studies are needed to determine whether the mutation we observed was caused by secondary genes necessary for testis determination.
In conclusion, we reported for the first time a case of 78, XY ovotesticular DSD in a cloned wolf. However, the specific reasons for this abnormality were not completely clarified in this study. Although the SRY gene is a primary factor for male sex determination, involvement of an additional gene needs to be established or ruled out. Therefore, further studies of other mutations arising in genes associated with the sex determination pathway should be undertaken. Such studies may provide clues for understanding the nature of intersexuality in mammals as well as mechanisms underlying gonadal development.
Figures and Tables
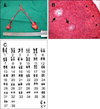 | Fig. 1SRY-positive 78, XY ovotesticular disorder of sex development in a cloned gray wolf. (A) Image of the ovary-like and uterus structures. (B) Histopathology of the ovotestis in the wolf. Note the hypoplastic seminiferous tubules (arrows) and undifferentiated primitive ovarian follicles (arrowheads). H&E stain. Scale bar = 100 µm. (C) Karyotype determined from skin fibroblasts collected for chromosome analysis. No chromosomal abnormalities were observed. |
Acknowledgments
We thank Dr. Barry D. Bavister (University of Wisconsin Madison, USA) and Dr. Byung Il Yoon (Kangwon National University, Korea) for assistance with preparing this manuscript. This study was financially supported by the Korean Ministry of Education, Science and Technology through the Korea Science and Engineering Foundation (No. 10033839-2011-13), the BK21 program for Veterinary Science, and the Seoul National University foundation (benefactor: RNL BIO, Korea), Hanwha L&C, Korea.
References
1. Beijerink NJ, Buijtels JJCWM, Okkens AC, Kooistra HS, Dieleman SJ. Basal and GnRH-induced secretion of FSH and LH in anestrous versus ovariectomized bitches. Theriogenology. 2007. 67:1039–1045.

2. De Lorenzi L, Groppetti D, Arrighi S, Pujar S, Nicoloso L, Molteni L, Pecile A, Cremonesi F, Parma P, Meyers-Wallen V. Mutations in the RSPO1 coding region are not the main cause of canine SRY-negative XX sex reversal in several breeds. Sex Dev. 2008. 2:84–95.

3. Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Münsterberg A, Vivian N, Goodfellow P, Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990. 346:245–250.

4. Jurka P, Galanty M, Zielinska P, Max A, Sysa P. Hypospadias in six dogs. Vet Rec. 2009. 164:331–333.

5. Lee BC, Kim MK, Jang G, Oh HJ, Yuda F, Kim HJ, Hossein MS, Kim JJ, Kang SK, Schatten G, Hwang WS. Dogs cloned from adult somatic cells. Nature. 2005. 436:641.

6. Meyers-Wallen VN. Genetics, genomics, and molecular biology of sex determination in small animals. Theriogenology. 2006. 66:1655–1658.

7. Oh HJ, Kim MK, Jang G, Kim HJ, Hong SG, Park JE, Park K, Park C, Sohn SH, Kim DY, Shin NS, Lee BC. Cloning endangered gray wolves (Canis lupus) from somatic cells collected postmortem. Theriogenology. 2008. 70:638–647.

8. Pajares G, Balseiro A, Pérez-Pardal L, Gamarra JA, Monteagudo LV, Goyache F, Royo LJ. Sry-negative XX true hermaphroditism in a roe deer. Anim Reprod Sci. 2009. 112:190–197.

9. Poth T, Breuer W, Walter B, Hecht W, Hermanns W. Disorders of sex development in the dog-Adoption of a new nomenclature and reclassification of reported cases. Anim Reprod Sci. 2010. 121:197–207.

10. Weng Q, Murase T, Asano M, Tsubota T. Male Pseudohermaphroditism in a raccoon dog (Nyctereutes procynoides). J Vet Med Sci. 2005. 67:603–605.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download