Abstract
Equine endometriosis is a multifactorial disease considered to be a major cause of equine infertility. The purpose of this study was to evaluate the reliability of histomorphological grading for biopsy-like samples compared to entire uterine wall samples, to examine the association between the degree of endometriosis with animal age, and to investigate the role of inflammation in endometriosis and the expression of different matrix metalloproteinases in equine endometrium. Histomorphological lesions in 35 uterine samples were examined while comparing biopsy-like samples and entire-wall samples. Seventeen uterine samples were stained with antibodies against MMP-2, MMP-9, MMP-14, and TIMP-2. The morphologic evaluation results of the biopsy-like tissue and entire-wall samples were significantly correlated. Endometriosis in older mares (>12 years of age) was more severe than in young mares (2~4 years of age), confirming the positive correlation between animal age and disease severity, while inflammation was poorly related to the degree of endometriosis. MMP-2 and MMP-14 were detected in stromal cells, while MMP-9 and TIMP-2 were both found in stromal and glandular epithelial cells. There were no significant differences in MMPs expression between the two groups (young vs. old mares). Additional studies on the activity of MMPs could further define the role of these enzymes in equine endometriosis.
Chronic endometrial degeneration, also known as equine endometriosis, is a common disease in older mares (>12 years of age) and is associated with decreased fertility, early embryonic death, and abortion [9]. Until now, little has been known about the etiology and pathogenesis of this major cause of equine infertility [11]. Endometriosis is a multi-factorial disease. Age, repeated pregnancies, parturition, chronic inflammation, and endocrine problems are all factors which seem to play a determinant role in the onset and severity of endometriosis [14].
Based on histopathological findings, this disease has been defined as a progressive process involving endometrial gland changes (cystic dilation, and atrophy or hypertrophy of the epithelium) associated with periglandular and/or stromal fibrosis [6,11,28]. In addition to this, signs of chronic inflammation and atrophy are frequently observed [13]. However, no study has actually determined the role of inflammation in endometriosis and extracellular matrix (ECM) re-modeling.
Matrix metalloproteinase (MMPs) are calcium- and zinc-dependent proteases believed to be responsible for the degradation and removal of ECM from the tissue [20]. With the exception of membrane MMPs, all these enzymes are secreted as inactive pro-enzymes and activated in the extracellular space by various factors including MMPs, plasmin, interleukin-1 beta, tumor necrosis factor-alpha, and other mediators [26,27]. Tissue inhibitors of metalloproteinase (TIMPs) are specific endogenous enzymes involved in controlling the local activities of MMPs in tissues [26]. MMPs play a vital role in many physiological and pathological processes in humans including embryogenesis, tissue remodeling, angiogenesis, wound healing, and metastasis [19,26]. Among the different MMPs, MMP-2 and MMP-9 (also known as gelatinases A and B, respectively) are the most widely studied because of their ability to degrade type IV collagen in the basement membrane and fibrillar collagens. Based on their ability to degrade basement membranes, various studies have reported increased levels of expression and activity for both MMP-2 and MMP-9 during different stages of organ development and under various pathological conditions, all situations in which dynamic tissue remodeling takes place [1,2,20]. MMP-2 is activated in two steps. First, the latent MMP-2 precursor is cleaved by MMP-14 to produce an intermediate form of MMP-2. This molecule then auto-catalytically converts itself into mature MMP-2 [26]. Increased expression of MMP-14 activates MMP-2 on the cell surface, a process that is required for cell invasion when localized in the invadopodia of human melanoma cells. In the ECM, TIMPs tightly regulate the activity of MMPs. TIMP-2 plays a dual role in controlling MMP-2 activation. First, the TIMP-2/MMP-14 complex is necessary for initiation of the activation process of pro-MMP-2. In addition, TIMP-2 binds to the active site of MMP-2, thereby inhibiting its activity [26].
In horses, it has been proposed that MMPs could have a role in different pathological processes. Clutterbuck et al. [4] have reported the involvement of MMPs in laminitis, osteoarthritis, recurrent airway obstruction, skin wounds repair, degenerative diseases of the central nervous system, ulcerative keratitis, and cancer. To our knowledge, only one study [27] has reported on the expression and activity of MMP-2 in equine endometriosis.
The objectives of the present study were to: 1) evaluate the reliability grading biopsy-like samples compared to entire uterine wall samples; 2) examine the relationship between histomorphological grading and animal age; 3) define the role of inflammation in endometrosis, and 4) immunohistochemically characterize MMP-2, MMP-9, MMP-14, and TIMP-2 expression in the uterus of mares according to the grade of endometriosis.
Endometrial specimens were obtained from a total of 35 mares of various breeds and with unknown reproductive histories. The mares were slaughtered between May and October (Pantano Carni s.r.l., Italy) and divided into two groups according to age: 2 to 4 years old (n = 20) and 12 years or older (n = 15). From each animal two samples were collected from the anterior uterine body: a biopsy-like sample representative of the mucosa (10~20 × 3 × 3 mm), and a larger sample (2 cm3) comprising of the entire wall. Stage of the oestrous cycle was determined by gross and histological examinations based on the morphological criteria of endometrial differentiation [13].
Samples were fixed in 4% buffered formalin, embedded in paraffin, cut into 4-µm sections, and stained with hematoxylin and eosin. Endometrial fibrosis in the biopsies was identified in sections stained with Masson's trichrome according to the grading system proposed by Kenney and Doig [13] that includes four categories: I, IIa, IIb, and III. Inflammation was quantified according to the total number of inflammatory cells in 10 high power fields (HPF) (×400). The samples were graded as follows: grade I, less then 100 inflammatory cells; grade II A, between 100 and 150 cells; grade II B, between 150 and 200 cells; and grade III more than 200 inflammatory cells. Additionally, severity of the score was upgraded by one stage when the degree of exocytosis was greater then 15 in 10 HPF (×400).
Samples of the entire uterine wall were evaluated. Serial paraffin sections were cut (4-µm thick) and placed onto slides (SuperFrost Plus; Menzel-Gläser, Germany). The slides were then incubated at 37℃ for 30 min before immunostaining was performed with an automatic immunostainer (Ventana Benchmark XT; Roche, Switzerland). The immunostainer used a secondary antibody with a horseradish peroxidase-conjugated polymer to enhance the signal (UltraViews Universal DAB; Ventana Medical System, USA). All reagents are automatically dispensed except for the primary antibody, which was dispensed by hand. The slides were incubated with antibodies against MMP-2, MMP-9, MMP-14, and TIMP-2 for 32 min at 42℃ (Table 1). Negative controls were incubated with diluent instead of primary antibody; plasma cells in the tissue controls were used as internal positive control. The immunohistochemical staining results were divided into the following four categories based on staining intensity: negative, 0 = no stained cells; 1 = weak staining; 2 = moderate staining; and 3 = intense staining. Immunostaining was blindly scored by two independent observers (L.A. and S.B.), and discordant scores were re-evaluated. The consensus scores were used for further analysis.
The extent and distribution of immunoreactivity specific for MMP-2, MMP-9, and MMP-14 were quantified as follows. At first, the number of stromal cells in 10 HPF (×400) in two horses from each group (control and older mares) was measured. Subsequently, a group mean value was obtained. Immunoreactivity specific for the different markers was expressed as number of labeled cells/the mean number of stromal cells. Finally, the data were expressed as a percentage. For TIMP-2, evaluation of immunolabeling was only qualitative.
All histochemical and immunohistochemical data were analyzed with an ANOVA using the GLM procedure and a correlation analysis with the CORR procedure of SAS (ver. 9.1; SAS, USA). A Chi-square test was used to compare the proportions of the histochemical data between the two different age groups and groups in different phases of the reproductive cycle. p-values less than 0.05 were considered to be significant for any statistical test performed.
This part of the study evaluated a total of 35 animals. According to the histomorphological features, 25 of the mares were in oestrus while and 10 were in diestrus. According to the classification of endometriosis established by Kenney and Doig, 19 of the 20 specimens from young mares were classified as grade I (Fig. 1, A1 and 2) and one animal was grade IIA (Fig. 1, B1 and 2). Among the older mares (n = 15), two cases were graded as IIA, six cases were grade IIB (Fig. 1, C1 and 2), and seven cases were grade III (Fig. 1, D1 and 2).
In young mares, the endometrial glands had a normal to slightly degenerated appearance with few multifocal fibrotic periglandular nests. Glandular ectasia was moderate. Multifocal inflammation was detected in the sub-epithelial layer, and was mainly characterized by the presence of lymphocytes and plasma cells with occasional eosinophils and neutrophils. In older mares, the glands were multifocally ectatic and surrounded by a thick layer of connective tissue. Glandular fibrotic nests were haphazardly distributed with numbers ranging from 1 to 10 per HPF and increasing under more severe fibrotic conditions. Moderate to severe multifocal lymphoplasmacytic infiltrate was found in the lamina propria. No differences in exocytosis were observed between the two groups.
Morphologic evaluation and grading of endometriosis for the biopsy-like tissue and entire-wall samples were highly correlated (r = 0.92; p < 0.001). Inflammation grade was poorly correlated with the severity of endometriosis for the entire-wall and biopsy-like samples (r2 = 0.47 and r2 = 0.39, respectively).
The expression patterns of MMP-2, MMP-9, MMP-14, and TIMP-2 were evaluated with immunohistochemistry; localization and staining intensity were observed. Data for this study are shown in Table 2. All specimens showed a highly granular staining pattern for MMP-9 in the cytoplasm of epithelial cells in the uterine glands and rarely in the luminal epithelium (Fig. 2B). The number of positive stromal cells was low (< 1%) and staining intensity was low or moderate except for two cases (5 and 13) that showed 9.5 and 9.2% of positive cells with high intensity staining. No differences were observed in the expression of MMP-9 according to reproductive stage. Differences in the staining patterns for MMP-2 were observed between older mares and the control animals (young mares). In normal endometrium (control), stromal cells in the stratum compactum were moderately stained. In the older mares, stromal cells in both the stratum compactum and stratum spongiosum showed variable immunoreactivity (Table 2 and Fig. 2A). Glandular epithelium, stromal cells, and occasionally luminal epithelium were positive for MMP-14 with weak to moderate intensity in 16 cases (Fig. 2C). No statistical significant differences were observed among animals with different grades of endometriosis and expression of MMP-14. Immunoreactivity against TIMP-2 was found in 16 cases. Stromal cells along with glandular and luminal epithelium had moderately to strongly stained cytoplasm (Fig. 2D).
Endometriosis is recognized as a multifactorial disease [9] and many studies have shown that advanced age is one of the main predisposing factors [12,13,22]. Uterine biopsy is a routine test to evaluate the grade of endometriosis and mare fertility [13]. Despite the widespread use of this technique, recent studies have questioned the efficacy of such methods due to the low reliability of a single endometrial biopsy to accurately assess the grade of endometriosis [7,8]. Our results demonstrated that the grading systems established for biopsies and entire-wall samples coincide. Therefore, results from this study confirmed that biopsy can be an indispensable clinical diagnostic tool for evaluating the fertility of mares. In agreement with the literature, our study also demonstrated the existence of a relationship between animal age and the grade of endometriosis [6,22]. When evaluating fertility, the most predictive factors seem to be the number of fibrotic nests and fibrosis grade. Our study showed that these two parameters are positively correlated with animal age (p < 0.001).
We found that inflammation is poorly correlated with the grade of endometriosis. Endometrial degeneration and fibrosis are known to originate from repetitive inflammatory injuries [8,24]. Damage from chronic inflammation triggers a complex tissue reaction resulting in ECM deposition and accumulation within the interstitium. Fibrosis continues to progress even after the inflammatory process has ended [3]. Therefore, progression of the fibrotic process after a critical point becomes independent of inflammation despite the presence of inflammation preceding fibrosis that affects the pathogenesis of endometriosis. This explains the low correlation between inflammation and endometriosis grade observed in the present study. Our finding is concordant with the results of a previous report showing that endometritis is independent of the degree of periglandular fibrosis [11]. It has been reported that induced endometritis is associated with fibrotic stromal cells activation 5 days post-infection, but over the 2-year period of the experiment the degree of endometriosis did not change [11]. In our investigation, statistical analysis showed that the degree of inflammation presents little correlation with endometrosis (p < 0.05), but it remains an indispensable parameter for the diagnosis.
We also analyzed the expression and distribution of different MMPs using immunohistochemistry. To the best of our knowledge, this is the first study to investigate different MMPs in equine endometrium. MMP-9, also known as gelatinase B, principally degrades collagen IV, the main component of basement membranes. In humans, MMP-9 has been reported to be expressed in inflammatory cells as well as glandular and periglandular stromal cells in the endometrium [17,23]. In our study, control luminal epithelial cells were positive for MMP-9 in normal equine endometrium. With different grades of endometriosis, increasing amounts of collagen IV are deposed around the endometrial glands and fibrotic nests [28]. In our study, we hypothesized that endometriosis increased the production of MMP-9 as a cellular reaction to degraded collagen IV. However, the expression of MMP-9 did not significantly differ between mares with endometriosis and the control horses. MMP-9 is reported to be involved in inflammatory processes and seems to be more involved in damage occurring during the early stages than chronic conditions [4]. In horses as well as humans, MMP-9 is principally produced by leucocytes and increased presence of this enzyme during acute inflammation is possibly associated with inflammatory cell migration during the earliest stages [5,15,16]. No previous studies have compared the expression of MMP-9 in healthy and fibrotic equine endometrial tissue. On one hand, MMP-9 expression and activity could not increase with endometriosis, leading to ECM accumulation and periglandular fibrosis. On the other hand, it is possible that MMP-9 is a marker of inflammation rather than fibrosis since expression of this enzyme is mainly associated with inflammatory processes rather than fibrotic conditions.
In the present study, MMP-2 (gelatinase A) expression was not significantly increased in diseased horses. Walter et al. [27] reported that the overall MMP-2 expression in normal or mildly affected endometrium was one third of that observed in severely affected tissues. Moreover, they used gelatine zymography to determine that up to 58% of MMP-2 is active in healthy specimens whereas that this value increased to 76% in affected samples. Likewise, Walter et al. [27] noted a difference in MMP-2 expression relative to progesterone plasma concentrations. More specifically, they observed increased expression of the protein during di-oestrus. In the present study, the oestrus cycle did not influence MMP expression but further investigations are required to elucidate the interaction between steroid hormone production and MMP activity.
MMP-14 is a trans-membrane protease capable of degrading different ECM components such as collagen type I, II, and III as well as fibronectin and laminin [10]. The main interest in this enzyme is due to its ability to activate different proteases, particularly MMP-2 and MMP-9 [21]. Our study was designed to measure MMP-14 expression relative to MMP-2 and MMP-9 in equine endometrium. We observed immunostaining specific for MMP-14 only occasionally. Few reports on MMP-14 expression in the reproductive system have been published, and most are related to pregnancy and placentation [18,25]. More studies are necessary to explain the role of MMP-14 in equine endometriosis.
Similarly, no information about TIMP-2 in horse endometrium exists in the literature. In our study, TIMP-2 was highly expressed in all samples regardless age or uterine condition. This is consistent with reports in humans showing that expression of TIMP-2 does not change during different phases of the menstrual cycle [29]. TIMP-2 has a dual role. At low concentrations it binds to MMP-14 and indirectly activates MMP-2. At high concentrations, actually inhibits MMP-2 [20,26]. A quantitative study could better identify the different conditions under which TIMP-2 acts as an inhibitor or activator.
In conclusion, our result indicated that immunohistochemistry appears to not be useful for evaluating equine fertility in clinical practice. However, further studies are necessary to better understand the role of the metalloproteinases in the pathogenesis of endometriosis. It is of particular interest to measure the different concentrations of active and latent forms of MMPs through gelatine zimography and evaluation of gene expression.
Figures and Tables
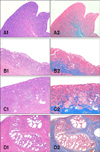 | Fig. 1Histopathology of the equine endometrial specimens. A1 and 2: grade I, B1 and 2: grade IIA, C1 and 2: grade IIB, D1 and 2: grade III. Specimens were classified according to Kenney and Doig [13] classification of endometriosis. Hematoxylin and eosin stain (A1, B1, C1, and D1) and Masson's trichrome stain (A2, B2, C2, and D2), ×40. |
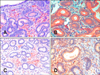 | Fig. 2Immunohistochemical staining of endometrial specimens from older mares with antibodies against MMP-2, MMP-9, MMP-14, and TIMP-2. (A) MMP-2 staining in inflammatory cells. (B) MMP-9 immunolabeling in glandular epithelium and stromal cells. (C) MMP-14 immunostaining in stromal cells. (D) TIMP immunostaining in glandular epithelium and periglandular stromal cells. A and C: ×200, B and D: ×400. |
Table 2
Results of MMP and TIMP-2 immunohistochemical analysis and endometriosis grade

*1~2: control cases, 3~17: older mares. †Grade according to the system proposed by Kenney and Doig [13] that includes four categories: I, IIa, IIb, and III. HPF: high power field.
References
1. Aresu L, Benali S, Garbisa S, Gallo E, Castagnaro M. Matrix metalloproteinases and their role in the renal epithelial mesenchymal transition. Histol Histopathol. 2011. 26:307–313.
2. Aresu L, Giantin M, Morello E, Vascellari M, Castagnaro M, Lopparelli R, Zancanella V, Granato A, Garbisa S, Aricò A, Bradaschia A, Mutinelli F, Dacasto M. Matrix metalloproteinases and their inhibitors in canine mammary tumors. BMC Vet Res. 2011. 7:33.

3. Cadario ME, Losinno L, Giguere S, Aguilar J, MacPherson M, Fitzpatrick C, Uhl E. Uterine expression of fibrogenic cytokines in the mare. Theriogenology. 2002. 58:449–452.

4. Clutterbuck AL, Harris P, Allaway D, Mobasheri A. Matrix metalloproteinases in inflammatory pathologies of the horse. Vet J. 2010. 183:27–38.

5. de Laat MA, Kyaw-Tanner MT, Nourian AR, McGowan CM, Sillence MN, Pollitt CC. The developmental and acute phases of insulin-induced laminitis involve minimal metalloproteinase activity. Vet Immunol Immunopathol. 2011. 140:275–281.

6. Doig PA, McKnight JD, Miller RB. The use of endometrial biopsy in the infertile mare. Can Vet J. 1981. 22:72–76.
7. Dybdal NO, Daels PF, Couto MA, Hughes JP, Kennedy PC. Investigation of the reliability of a single endometrial biopsy sample, with a note on the correlation between uterine cysts and biopsy grade. J Reprod Fertil Suppl. 1991. 44:697.
8. Fiala SM, Esmeraldino A, Jobim MIM, Garbade P, Wolf CA, Richter G, Gregory RM, Mattos RC. Endometrial fibrotic changes. Is one biopsy enough to diagnose degenerative changes? Anim Reprod Sci. 2010. 121:Suppl. S89–S90.
9. Flores JM, Rodríguez A, Sánchez J, Gómez-Cuétara C, Ramiro F. Endometrosis in mares: incidence of histopathological alterations. Reprod Domest Anim. 1995. 30:61–65.

10. Giantin M, Aresu L, Benali S, Aricò A, Morello EM, Martano M, Vascellari M, Castagnaro M, Lopparelli RM, Zancanella V, Granato A, Mutinelli F, Dacasto M. Expression of matrix metalloproteinases, tissue inhibitors of metalloproteinases and vascular endothelial growth factor in canine mast cell tumours. J Comp Pathol. 2012. Epub ahead of print. doi:10.1016/j.jcpa.2012.01.011.

11. Hoffmann C, Ellenberger C, Mattos RC, Aupperle H, Dhein S, Stief B, Schoon HA. The equine endometrosis: new insights into the pathogenesis. Anim Reprod Sci. 2009. 111:261–278.

12. Kenney RM. Cyclic and pathologic changes of the mare endometrium as detected by biopsy, with a note on early embryonic death. J Am Vet Med Assoc. 1978. 172:241–262.
13. Kenney RM, Doig PA. Morrow DA, editor. Equine endometrial biopsy. Current Therapy in Theriogenology. 1986. 2nd ed. Philadelphia: Saunders;723–729.
14. Kenney RM, Ganjam VK. Selected pathological changes of the mare uterus and ovary. J Reprod Fertil Suppl. 1975. 335–339.
15. Loftus JP, Belknap JK, Black SJ. Matrix metalloproteinase-9 in laminae of black walnut extract treated horses correlates with neutrophil abundance. Vet Immunol Immunopathol. 2006. 113:267–276.

16. Loftus JP, Johnson PJ, Belknap JK, Pettigrew A, Black SJ. Leukocyte-derived and endogenous matrix metalloproteinases in the lamellae of horses with naturally acquired and experimentally induced laminitis. Vet Immunol Immunopathol. 2009. 129:221–230.

17. McCawley LJ, Matrisian LM. Matrix metalloproteinases: they're not just for matrix anymore! Curr Opin Cell Biol. 2001. 13:534–540.

18. Mishra B, Kizaki K, Koshi K, Ushizawa K, Takahashi T, Hosoe M, Sato T, Ito A, Hashizume K. Expression of extracellular matrix metalloproteinase inducer (EMMPRIN) and its related extracellular matrix degrading enzymes in the endometrium during estrous cycle and early gestation in cattle. Reprod Biol Endocrinol. 2010. 8:60.

19. Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004. 16:558–564.

20. Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006. 69:562–573.

21. Osenkowski P, Toth M, Fridman R. Processing, shedding, and endocytosis of membrane type 1-matrix metalloproteinase (MT1-MMP). J Cell Physiol. 2004. 200:2–10.

22. Ricketts SW, Alonso S. The effect of age and parity on the development of equine chronic endometrial disease. Equine Vet J. 1991. 23:189–192.

23. Skinner JL, Riley SC, Gebbie AE, Glasier AF, Critchley HO. Regulation of matrix metalloproteinase-9 in endometrium during the menstrual cycle and following administration of intrauterine levonorgestrel. Hum Reprod. 1999. 14:793–799.

24. Troedsson MHT. Robinson NE, editor. Diseases of the uterus. Current Therapy in Equine Medicine. 1997. 4th ed. Philadelphia: Saunders;517–523.
25. Uekita T, Tanaka SS, Sato H, Seiki M, Tojo H, Tachi C. Expression of membrane-type 1 matrix metalloproteinase (MT1-MMP) mRNA in trophoblast and endometrial epithelial cell populations of the synepitheliochorial placenta of goats (Capra hircus). Arch Histol Cytol. 2001. 64:411–424.

26. Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003. 92:827–839.
27. Walter I, Handler J, Miller I, Aurich C. Matrix metalloproteinase 2 (MMP-2) and tissue transglutaminase (TG 2) are expressed in periglandular fibrosis in horse mares with endometrosis. Histol Histopathol. 2005. 20:1105–1113.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download