Abstract
To examine the genetic background of avian pathogenic Escherichia coli (APEC) that affects virulence of this microorganism, we characterized the virulence genes of 101 APEC strains isolated from infected chickens between 1985~2005. Serotypes were determined with available anti-sera and median lethal doses were determined in subcutaneously inoculated chicks. The virulence genes we tested included ones encoding type 1 fimbriae (fimC), iron uptake-related (iroN, irp2, iucD, and fyuA), toxins (lt, st, stx1, stx2, and vat), and other factors (tsh, hlyF, ompT, and iss). Twenty-eight strains were found to be O1 (2.0%), O18 (3.0%), O20 (1.0%), O78 (19.8%), and O115 (2.0%) serotypes. The iroN (100%) gene was observed most frequently followed by ompT (94.1%), fimC (90.1%), hlyF (87.1%), iss (78.2%), iucD (73.3%), tsh (61.4%), fyuA (44.6%), and irp2 (43.6%). The strains were negative for all toxin genes except for vat (10.9%). All the strains were classified into 27 molecular pathotypes (MPs). The MP25, MP19, and MP10 pathotypes possessing iroN-fimC-ompT-hlyF-iucD-tsh-iss-irp2-fyuA (22.8%), iroN-fimC-ompT-hlyF-iucD-tsh-iss (21.8%), and iroN-fimC-ompT-hlyF-iss (11.9%) genotypes, respectively, were predominant. Redundancy of iron uptake-related genes was clearly observed and some strains were associated with higher mortality than others. Therefore, strains with the predominant genotypes can be used for diagnosis and vaccine.
Extra-intestinal infection with avian pathogenic Escherichia (E.) coli (APEC) induces colibacillosis in chickens, a disease characterized by polyserositis, septicemic shock, and cellulitis [8]. Out of 173 E. coli serogroups, the most commonly encountered in APEC are O1, O2, O35, and O78 [2,31] although the order of prevalence varies in different countries and farms [6]. E. coli are divided four main phylogenetic groups: A, B1, B2, and D. Among these, B2 and D are the most common among human pathogenic E. coli while A is most frequently found in APEC [4,19,22].
Recently, various virulence genes were identified in APEC, and their distribution and frequency among APEC isolates have been reported [13,14,21]. These virulence genes may play roles in various aspects of the extra-intestinal pathogenesis of APEC, and their functions can be categorized as adhesion, iron acquisition, hemolysis, protection from bactericidal host factors, and toxin production [12]. Type 1 fimbriae mediate E. coli adherence to host epithelial cells of the respiratory tract for colonization. Expression of fimC is important for fimbrial assembly and anchoring of the assembled fimbriae [23,35]. Temperature-sensitive hemagglutinin (tsh) is involved in adherence to the avian respiratory tract, and is primarily responsible for the development of airsacculitis and colisepticemia [11,27,36]. Invasive strains of E. coli have iron uptake systems that compete with host transferrin for available iron molecules [25,40]. Iron uptake chelate gene D (iucD) is involved in the biosynthesis of aerobactin and APEC virulence [30]. Ferric yersiniabactin uptake A (fyuA) and iron-repressible protein 1 and 2 (irp2), which are involved in iron acquisition in Yersinia, are found in human E. coli and APEC strains [16,37]. The outer membrane siderophore receptor gene iroN, which was first reported in Salmonella enterica, affects the virulence of APEC [3,10]. The increased serum survival gene iss is associated with APEC complement resistance [34]. A new class of hemolysin, hlyF, was identified in E. coli strains from broilers and reportedly influences the virulence of APEC [20,29]. OmpT, which cleaves the antimicrobial peptides protamine and plasminogen, was identified in human E. coli recovered from patients with urinary tract infections and APEC [20,26,38]. Heat-labile and heat-stable toxins (LT and ST, respectively) along with shiga toxins (stx1 and stx2) were found in pathogenic E. coli, and vacuolating autotransporter toxin (vat) was identified in APEC [33]. The frequency of various virulence genes in APEC strains has been reported, but knowledge about the frequencies of combined virulence genes and patterns of virulence gene accumulation in APEC strains is limited, which diminishes the understanding of how APEC evolution affects pathogenicity [12]. To address this shortcoming, we identified the serotypes and phylogenetic groups of 101 APEC strains collected in Korea from 1985~2005. We also measured the frequencies of virulence genes fimC, tsh, hlyF, iroN, iucD, fyuA, irp2, iss, ompT, vat, lt, st, stx1, and stx2. These genes were divided into 27 molecular pathotypes (MPs). Our findings provided information about the hypothetical steps involved in virulence gene acquisition according to the virulence gene combinations and MP frequencies. Finally, we determined the media lethal dose (LD50) of some APEC strains in chickens to explore the correlation MP and APEC virulence.
One hundred and one APEC strains were isolated between 1985~2005 from chickens in Korea suffering from colibacillosis. All of the APEC isolates were identified using VITEK Gram-Negative Identification (GNI) Cards (bioMerieux Vitek, USA) as previously described [24]. Commercial male brown layer chicks from healthy breeders without a history of colibacillosis (Yangji hatchery, Korea) were used for experiments measuring the LD50.
O-serotyping was performed as previously described [15] using commercial antisera against O1, O6, O8, O15, O18, O20, O78, and O115 (Denka Seiken, Japan). E. coli strains were cultured on tryptic soy agar (Becton Dickinson, USA) plate at 37℃ overnight. Each cultured strain was suspended in sterilized saline at the concentration of 1.8 × 109 colony forming unit (CFU)/mL, and divided into two screw-capped test tubes. The one was boiled at 100℃ for 1 h and the other was autoclaved for 2.5 h. After cooling of the test tubes formaldehyde and crystal violet (Sigma-Aldrich, USA) were added to become 0.5% (v/v) and 0.005% (w/v), respectively. The reference antiserum was 160-fold diluted with sterilized saline containing 1% sodium azide (w/v; Sigma-Aldrich, USA) and 50 µL was mixed with 50 µL of E. coli antigen prepared above in the v-bottomed 96-well polystyrene microtiter plate (Sigma-Aldrich, USA). After incubation at 37℃ overnight the pinpoint and larger congregated precipitates were read as negative and positive, respectively.
E. coli phylogenetic grouping was accomplished by a rapid and simple method as previously described [5] with modifications of some primers as shown in Table 1. Nucleotide sequences of the previously described primers were compared to the genome sequences of E. coli in GenBank using a BLAST search (NCBI, USA). As shown in Table 1, the YjaAF, YjaAR, and TspE4C2R primers were modified to bind to the variable nucleotides [e.g. YjaAF (the fifth nucleotide from the 3'-end)/YjaAR (the fourth nucleotide from the 5'-end), IHE3034 strain (CP001969); TspE4C2R (the fifth nucleotide from the 5'-end), IAI1 strain (NC_011741)]. The both chuA and TspE4.C2 negative and positive E. coli strains were grouped into group A and B2, respectively, and the chuA-negative and TspE4.C2-positive, and the chuA-positive and yjaA-negative E. coli strains were grouped into B1 and D, respectively.
The virulence genes fimC, tsh, hlyF, iroN, iucD, fyuA, irp2, iss, ompT, vat, lt, st, stx1, and stx2 were detected by PCR. The primer sets used for this procedure are listed in Table 1. MPs were determined according to the combinations of virulence genes observed.
DNA of APEC strain was extracted using a G-spin for Bacteria kit (iNtRON Biotechnology, Korea) according to the manufacturer's instructions. The PCR conditions of phylogenetic typing and molecular pathotyping were same. Briefly, the PCR solution was composed of 10 × buffer (2 µL; MACROGEN, Korea), dNTPs (2.5 mM, 0.4 µL; MACROGEN, Korea), forward and reverse primers (10 pmol/µL, 0.5 µL each; MACROGEN, Korea), Taq DNA polymerase (5 U/µL, 0.2 µL; MACROGEN, Korea), distilled water (15.4 µL), and template DNA (50 ng/µL, 1 µL). The cycling conditions were as follows: 94℃ for 3 min followed by 35 cycles of 94℃ for 30 sec; 55℃ for 30 sec; and 72℃ for 1 min; and a final extension step at 72℃ for 5 min. Amplicons were separated by electrophoresis in 1.0% agarose gels with a 1-kb ladder as the molecular size marker (iNtRON Biotechnology, Korea).
Eleven APEC strains were selected for further analysis based on MP and serotype. These included E9 (MP27/B2), E22 (MP24/O18/B2), E29 (MP13/B1), E30 (MP25/O78/A), E43 (MP7/O78/B2), E64 (MP26//B2), E89 (MP25/D), E104 (MP23/O78/B1), E115 (MP19/O78/A), E129 (MP20/O1), and E138 (MP19/A). The strains were classified according to virulence based on lethality in 7-day-old chicks for 7 days following subcutaneous inoculation as previously described [11,18]. Lethality classes (LC) were defined as previously described [11]: LC1, LD50 less than or equal to 5 × 106 CFU; LC2, LD50 is from 5 × 106 to 108 CFU; and LC3, LD50 greater than or equal to 5 × 108 CFU.
The frequencies of virulence genes were compared between the periods (1985~1988, 1990~1999, and 2000~2005) with Chi-square and Fisher's exact tests (95% confidence interval) using SPSS for Windows (ver. 12.0; SPSS, USA). The p-values less than 0.05 were considered as significant.
One hundred and one APEC strains were serotyped with antisera specific for O1, O6, O8, O15, O18, O20, O78, and O115. Serotype O78 was the most frequently observed (19.8%, 20/101) followed by O18 (3.0%, 3/101), O1 (2.0%, 2/101), O115 (2.0%, 2/101), and O21 (1.0%, 1/101).
Eighty-six Korean APEC strains were divided into different phylogenetic groups. Group A was the largest (39.5%, 34/86), groups B1 (23.3%, 20/86) and B2 (22.1%, 19/86) were similar in size, and group D (15.1%, 13/86) was notably smaller (Table 2).
The frequencies and combinations of virulence genes for the 101 APEC strains were assessed (Tables 1 and 3). IroN was carried by all of the APEC strains while 90.1, 94.1, 87.1, 78.2, 73.3, 61.4, 44.6, 43.6, and 10.9% of the strains carried fimC, ompT, hlyF, iss, iucD, tsh, fyuA, irp2, and vat, respectively (Table 3). The lt, st, stx1, and stx2 toxin genes were not detected in any strain. Chronological increases of iss and fyuA/irp2 frequencies between 1985~1988 and 2000~2005, 1985~1988 and 1990~1999/2000~2005, and 1985~1988 and 2000~2005 were significant (p < 0.05, Table 3).
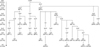
The 101 APEC strains were divided into 27 MPs based on the different combinations of virulence genes. Over half of the APEC strains (56.5%) were classified as MP25 (22.8%, iroN-fimC-ompT-hlyF-iucD-iss-fyuA-irp2-tsh), MP19 (21.8%, iroN-fimC-ompT-hlyF-iucD-iss-tsh), or MP10 (11.9%, iroN-fimC-ompT-hlyF-iss). The frequencies of iroN, fimC, ompT, hlyF, iucD, iss, fyuA, irp2, tsh, and vat among all of the MPs were 100, 70.4, 63.0, 59.3, 59.3, 59.3, 51.9, 48.1, 33.3, and 14.8%, respectively. Comparing the virulence gene frequencies of the APEC strains and the MPs, the frequencies of fimC, ompT, hlyF, iss, iucD, and tsh were lower according to MPs compared to the APEC strains. In contrast, the frequencies of fyuA, irp2, and vat were higher according to the MPs compared to the APEC strains (Table 3). Virulence gene profiles of the MPs showed a cumulative pattern, and the hypothetical steps of virulence gene acquisition were illustrated according to virulence gene frequencies among the MPs (Fig. 1). We hypothesized that the probability of virulence gene transmission is similar each other, and genes with higher frequencies would be introduced earlier into the APEC strains than other genes with lower frequencies. MP1 microorganisms, which had acquired hlyF, evolved into MP8 and MP12 strains by acquiring iss and iucD-iss, respectively, and MP12 further evolved into MP21 by acquiring fyuA-irp2. MP1 organisms, which had not acquired hlyF but rather iucD, later evolved into MP16 and MP17 through the acquisition of tsh and iss-fyuA-irp2, respectively. APEC strains that had not gained fimC and ompT evolved into MP18 and MP19 by acquiring hlyF-iucD-iss-fyuA-irp2 and iucD-fyuA-irp2, respectively. APEC strains possessing iroN and fimC evolved into MP2, MP3, and MP7 by gaining ompT, hlyF, and iucD-iss, respectively. MP2 evolved into MP5, MP15, MP11, and MP4 through the acquisition of tsh, iss-fyuA-irp2, fyuA-irp2, and hlyF, respectively. MP15 further transformed into MP20 by gaining vat. MP4 acquired iss to become MP10. MP4 organisms that acquired iucD evolved into MP13, MP23, and MP14 by acquiring tsh, fyuA-irp2-tsh, and iss, respectively. MP23 further transformed into MP24 by gaining vat. MP14 evolved into MP19, MP22, MP25, and MP26 by acquiring tsh, fyuA-tsh, irp2-tsh, and fyuA-irp2-vat, respectively. Finally, MP25 further developed into MP27 via the acquisition of vat.
According to LD50 determination, the 11 tested strains were classified as LC1 (n = 2), LC2 (n = 3), and LC3 (n = 6). E64 (MP26/B2) and E89 (MP25/D) were designated as LC1. E22 (MP24/O18/B2), E104 (MP23/O78/B1), and E138 (MP19/A) were found to be LC2. Finally, E9 (MP2/B2), E29 (MP13/B1), E30 (MP25/O78/A), E43 (MP7/O78/B2), E115 (MP19/O78/A), and E129 (MP20/O1) were determined to be LC3. Although E43 and E115 were classified as LC3, these strains were the causative agent of polyserositis, or polyserositis and cellulitis in some of the surviving chickens.
APECs have diverse serotypes, but certain ones (O78, O2, and O1) are more frequently observed that others. The frequencies of O78 among APEC isolates vary according to location and host. In one study [13], O78 was the second most frequently observed serotype in Germany (14.7%) after O2 (28.7%). These two serotypes were the most frequent (45.6 and 20%) in Ireland [28] and turkeys in the United States [1]. Although we tested for serotypes with only a limited number of antisera, we determined that O78 was also the most frequent serotype (19.8%) in Korea.
Previous studies have reported the frequencies of A, B1, B2, and D groups in APEC strains as 34.5~71.0, 4.1~21.3, 7.9~44.5, and 12.0~29.9%, respectively [9,22]. The B2 group is closely related to human extra-intestinal pathogenic E. coli (ExPEC), and is frequently found among human uropathogenic and neonatal meningitis E. coli strains [4,22]. Therefore, further studies on the correlation between B2 group APEC isolates and human ExPEC strains may be valuable for examining zoonosis potential. Recently, we identified mutations in yjaA.1 (the fifth C to T from the 3' end), yjaA.2 (the fourth G to A from the 5' end), and TspE4.C2 (the fifth C to T from the 5' end and the third A to G from the 3' end) using genome sequences derived from 17 strains of E. coli and comparing the nucleotide sequences of phylogenetic grouping primer sets [5]. These mutations may have occasionally been the cause weak signals or false negatives for yjaA and TspE4.C2, resulting in artificially increased frequencies for the A and D groups. Therefore, modified forward and reverse primers for yjaA and reverse primer for TspE4.C2 may be able to minimize errors in phylogenetic grouping.
The frequencies of iroN, fimC, ompT, hlyF, iucD, iss, fyuA, irp2, tsh, and vat were reported to be 85.4~89.0, 90.4~92.7, 60.0~81.6, 0~81.7, 78.0~100, 38.5~100, 58.2~71.3, 68.0~100, 39.5~93.9, and 33.4~64.3%, respectively [7,13,14,20,22,32]. In the present study, the frequencies of fimC, hlyF, iucD, iss, and tsh were similar to the ones in these previous reports while the frequencies of iroN and ompT were higher, and the frequencies of irp2, fyuA, and vat were lower. Similarities in hlyF, iucD and iss, and fyuA and irp2 frequencies reflect possible co-transmission of these genes but there was no report on their co-transmission [20,37]. Although frequencies of virulence genes in APECs vary according to location and host, the presence of redundant iron uptake-related genes (iroN, chuA, iucD, fyuA, and irp2) is common among APEC strains. The roles of these redundant genes are unclear, but they are expected to have varying functions under different niche conditions. Considering the essential role of iron-uptake in APEC pathogenicity, various redundant iron uptake-related proteins may be useful for evading humoral immunity. The functions of virulence genes tested in the present study are well documented, and accumulation of these genes may be a potential risk factor for APEC infection. Therefore, monitoring MPs with multiple virulence genes in poultry farms and products along with comparative studies on MP distribution in different hosts may be helpful to decrease economic loss in the poultry industry and reduce the potential zoonotic risks of APECs [22].
To date, various methods have been tested for reproducing the clinical signs and pathological lesions caused by APECs. Animals were inoculated with APEC strains via intratracheal, intra-air sac, or subcutaneous routes with or without triggering infectious microbes or exposure to ammonia [11,17,18,39]. In the present study, we measured the LD50 of selected APEC strains by subcutaneously inoculating 7-day-old male brown layer chicks; the strains were classified as LC1, LC2, and LC3. High virulence of the E64 and E89 strains can be explained by their relatively abundant expression of virulence genes, but an absence of acute mortality due to infection with E9 and E30 (strains which carried virulence genes similar to E64 and E89) may reflect the involvement of other virulence genes. We only evaluated the mortality of chickens inoculated within 7 days; some of these birds developed polyserositis and cellulitis without increased mortality. Therefore, an extended observation period (i.e., 14 days) and grading of gross lesions in organs and tissues may improve the accuracy of virulence assays in the future.
In conclusion, our finding demonstrated that different virulence genes have accumulated in APEC strains. Furthermore, APEC microorganisms with the predominant genotypes can be used as diagnostic targets and colibacillosis vaccine candidates. Further studies on the biological significance of redundant iron uptake-related genes and presence of new genes associated with high mortality should be conducted.
Figures and Tables
Acknowledgments
This research was supported by the Technology Development Program (No. 105110-02-1-SB010) for Agriculture and Forestry, Ministry for Food, Agriculture, Forestry and Fisheries, and the Technique Innovation research program of Small and Medium Business Administration, Korea.
References
1. Altekruse SF, Elvinger F, DebRoy C, Pierson FW, Eifert JD, Sriranganathan N. Pathogenic and fecal Escherichia coli strains from turkeys in a commercial operation. Avian Dis. 2002. 46:562–569.

2. Barnes HJ, Vaillancourt JP, Gross WB. Saif YM, editor. Colibacillosis. Diseases of Poultry. 2003. 11th ed. Ames: Iowa State Press;631–656.
3. Bäumler AJ, Norris TL, Lasco T, Voight W, Reissbrodt R, Rabsch W, Heffron F. IroN, a novel outer membrane siderophore receptor characteristic of Salmonella enterica. J Bacteriol. 1998. 180:1446–1453.

4. Bingen E, Picard B, Brahimi N, Mathy S, Desjardins P, Elion J, Denamur E. Phylogenetic analysis of Escherichia coli strains causing neonatal meningitis suggests horizontal gene transfer from a predominant pool of highly virulent B2 group strains. J Infect Dis. 1998. 177:642–650.

5. Clermont O, Bonacorsi S, Bingen E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol. 2000. 66:4555–4558.

6. Cortes P, Blanc V, Mora A, Dahbi G, Blanco JE, Blanco M, Lopez C, Andreu A, Navarro F, Alonso MP, Bou G, Blanco J, Llagostera M. Isolation and characterization of potentially pathogenic antimicrobial-resistant Escherichia coli strains from chicken and pig farms in Spain. Appl Environ Microbiol. 2010. 76:2799–2805.

7. Delicato ER, de Brito BG, Gaziri LC, Vidotto MC. Virulence-associated genes in Escherichia coli isolates from poultry with colibacillosis. Vet Microbiol. 2003. 94:97–103.

8. Dho-Moulin M, Fairbrother JM. Avian pathogenic Escherichia coli (APEC). Vet Res. 1999. 30:299–316.
9. Dissanayake DRA, Wijewardana TG, Gunawardena GA, Poxton IR. Distribution of lipopolysaccharide core types among avian pathogenic Escherichia coli in relation to the major phylogenetic groups. Vet Microbiol. 2008. 132:355–363.

10. Dozois CM, Daigle F, Curtiss R 3rd. Identification of pathogen-specific and conserved genes expressed in vivo by an avian pathogenic Escherichia coli strain. Proc Natl Acad Sci USA. 2003. 100:247–252.

11. Dozois CM, Dho-Moulin M, Brée A, Fairbrother JM, Desautels C, Curtiss R 3rd. Relationship between the Tsh autotransporter and pathogenicity of avian Escherichia coli and localization and analysis of the tsh genetic region. Infect Immun. 2000. 68:4145–4154.

12. Dziva F, Stevens MP. Colibacillosis in poultry: unravelling the molecular basis of virulence of avian pathogenic Escherichia coli in their natural hosts. Avian Pathol. 2008. 37:355–366.

13. Ewers C, Janssen T, Kiessling S, Philipp HC, Wieler LH. Molecular epidemiology of avian pathogenic Escherichia coli (APEC) isolated from colisepticemia in poultry. Vet Microbiol. 2004. 104:91–101.

14. Ewers C, Janssen T, Kiessling S, Philipp HC, Wieler LH. Rapid detection of virulence-associated genes in avian pathogenic Escherichia coli by multiplex polymerase chain reaction. Avian Dis. 2005. 49:269–273.

15. Ewing WH, Lindberg AA. Bergan T, editor. Serology of Shigella. Methods in Microbiology. 1984. Vol. 14. London: Academic Press;114–142.
16. Gophna U, Oelschlaeger TA, Hacker J, Ron EZ. Yersinia HPI in septicemic Escherichia coli strains isolated from diverse hosts. FEMS Microbiol Lett. 2001. 196:57–60.

17. Goren E. Observations on experimental infection of chicks with Escherichia coli. Avian Pathol. 1978. 7:213–224.
18. Harry EG, Hemsley LA. The association between the presence of septicaemia strains of Escherichia coli in the respiratory and intestinal tracts of chickens and the occurrence of coli septicaemia. Vet Rec. 1965. 77:35–40.
19. Herzer PJ, Inouye S, Inouye M, Whittam TS. Phylogenetic distribution of branched RNA-linked multicopy single-stranded DNA among natural isolates of Escherichia coli. J Bacteriol. 1990. 172:6175–6181.

20. Johnson TJ, Siek KE, Johnson SJ, Nolan LK. DNA sequence of a ColV plasmid and prevalence of selected plasmid-encoded virulence genes among avian Escherichia coli strains. J Bacteriol. 2006. 188:745–758.

21. Johnson TJ, Wannemuehler Y, Doetkott C, Johnson SJ, Rosenberger SC, Nolan LK. Identification of minimal predictors of avian pathogenic Escherichia coli virulence for use as a rapid diagnostic tool. J Clin Microbiol. 2008. 46:3987–3996.

22. Johnson TJ, Wannemuehler Y, Johnson SJ, Stell AL, Doetkott C, Johnson JR, Kim KS, Spanjaard L, Nolan LK. Comparison of extraintestinal pathogenic Escherichia coli strains from human and avian sources reveals a mixed subset representing potential zoonotic pathogens. Appl Environ Microbiol. 2008. 74:7043–7050.

23. Jones CH, Pinkner JS, Nicholes AV, Slonim LN, Abraham SN, Hultgren SJ. FimC is a periplasmic PapD-like chaperone that directs assembly of type 1 pili in bacteria. Proc Natl Acad Sci USA. 1993. 90:8397–8401.

24. Kim TE, Jeong YW, Cho SH, Kim SJ, Kwon HJ. Chronological study of antibiotic resistances and their relevant genes in Korean avian pathogenic Escherichia coli isolates. J Clin Microbiol. 2007. 45:3309–3315.

25. Lafont JP, Dho M, D'Hauteville HM, Bree A, Sansonetti PJ. Presence and expression of aerobactin genes in virulent avian strains of Escherichia coli. Infect Immun. 1987. 55:193–197.

26. Lundrigan MD, Webb RM. Prevalence of ompT among Escherichia coli isolates of human origin. FEMS Microbiol Lett. 1992. 76:51–56.
27. Maurer JJ, Brown TP, Steffens WL, Thayer SG. The occurrence of ambient temperature-regulated adhesins, curli, and the temperature-sensitive hemagglutinin tsh among avian Escherichia coli. Avian Dis. 1998. 42:106–118.

28. McPeake SJW, Smyth JA, Ball HJ. Characterisation of avian pathogenic Escherichia coli (APEC) associated with colisepticaemia compared to faecal isolates from healthy birds. Vet Microbiol. 2005. 110:245–253.

29. Morales C, Lee MD, Hofacre C, Maurer JJ. Detection of a novel virulence gene and a Salmonella virulence homologue among Escherichia coli isolated from broiler chickens. Foodborne Pathog Dis. 2004. 1:160–165.

30. Ngeleka M, Kwaga JKP, White DG, Whittam TS, Riddell C, Goodhope R, Potter AA, Allan B. Escherichia coli cellulitis in broiler chickens: clonal relationships among strains and analysis of virulence-associated factors of isolates from diseased birds. Infect Immun. 1996. 64:3118–3126.

31. Orskov F, Orskov I. Escherichia coli serotyping and disease in man and animals. Can J Microbiol. 1992. 38:699–704.
32. Ozawa M, Harada K, Kojima A, Asai T, Sameshima T. Antimicrobial susceptibilities, serogroups, and molecular characterization of avian pathogenic Escherichia coli isolates in Japan. Avian Dis. 2008. 52:392–397.

33. Parreira VR, Gyles CL. A novel pathogenicity island integrated adjacent to the thrW tRNA gene of avian pathogenic Escherichia coli encodes a vacuolating autotransporter toxin. Infect Immun. 2003. 71:5087–5096.

34. Pfaff-McDonough SJ, Horne SM, Giddings CW, Ebert JO, Doetkott C, Smith MH, Nolan LK. Complement resistance-related traits among Escherichia coli isolates from apparently healthy birds and birds with colibacillosis. Avian Dis. 2000. 44:23–33.

35. Pourbakhsh SA, Dho-Moulin M, Brée A, Desautels C, Martineau-Doize B, Fairbrother JM. Localization of the in vivo expression of P and F1 fimbriae in chickens experimentally inoculated with pathogenic Escherichia coli. Microb Pathog. 1997. 22:331–341.

36. Provence DL, Curtiss R 3rd. Isolation and characterization of a gene involved in hemagglutination by an avian pathogenic Escherichia coli strain. Infect Immun. 1994. 62:1369–1380.

37. Schubert S, Rakin A, Karch H, Carniel E, Heesemann J. Prevalence of the "high-pathogenicity island" of Yersinia species among Escherichia coli strains that are pathogenic to humans. Infect Immun. 1998. 66:480–485.

38. Stumpe S, Schmid R, Stephens DL, Georgiou G, Bakker EP. Identification of OmpT as the protease that hydrolyzes the antimicrobial peptide protamine before it enters growing cells of Escherichia coli. J Bacteriol. 1998. 180:4002–4006.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download