Abstract
This study was done after identifying animals with a twisted carpal joint in goat herd. These included a kid goat walking on its articulus carpii and a newborn goat with a stiff leg. Necropsies of the diseased goats revealed swollen carpal joints that were twisted backwards. Arthritis was observed during microscopic examination of the carpal joints. Very low levels of eosinophil, leucocyte, and lymphocyte cell infiltration were found in the central nervous system and meninges. Serum copper levels were significantly decreased in most of the animals. All of these results led us to diagnose the animals with swayback disease.
Copper (Cu) is essential for fetal development, and maternal dietary Cu deficiency can have both short and long term consequences [7,9]. Copper deficiency can occur as congenital or acquired forms, and is often mistaken for caprine arthritis encephalitis virus (CAEV) infection. Clinical symptoms of Cu deficiency vary and include poor appetite in congenital forms,weakness of limbs, twisted joints, edema, head tremors, incoordination, ataxia, paresis, and paralysis [10,14,15]. Degeneration and necrosis of the motor neurons in the medulla spinalis and cerebellum as well as demyelinization were also reported in cases of Cu deficiency [6,10].
CAEV belongs to the lenti virus genus of the Retroviridea family [10]. This virus infects goats and has been isolated from synovial fluids, plexus corideus, lungs, lymphocytes, and colostrum of infected animals [11]. CAEV infection can be diagnosed by clinical and pathological findings of the anterior extremities (articulus carpii), nervous system, lungs, and breast [10]. Although the most important symptom is related with the central nervous system which is the leukoencephalomyelitis, CAEV infection also draws attention by being together with arthritis in goat kids.
This study was done after identifying twisted carpal joints in goats belonging to a single herd that a kid goat congenitally walking on its articulus carpii and a newborn goat that had a stiff leg. It was conducted in the Denizli region of South Western Turkey. After the claim of the owner of the goat herd from the Veterinary Faculty, the animals of the herd were examined during the delivery season. We observed twisted carpal joints and a newborn with a horse walk. The nine goats with congenital disease were transferred to the laboratory at Ankara University (Turkey), and the entire herd was observed under veterinary/scientific control. Nine goat kids out of the 37 diseased from 250 pregnant goats were necropsied and samples were taken for pathologic and microbiological examinations. Samples of the joints, lungs, and nervous system tissues were fixed with 10% neutral buffered formalin, embedded in paraffin, cut into sections that were 5~6 microns thick, and stained with hematoxylin and eosin.
For virological examination, synovial membrane and fluid along with lung and brain tissue samples were obtained from the same animals; primary calf kidney cells were cultured in inoculua and monitored for cytopathic effects (CPE). Poor CPE was observed at the blank fourth passage. Identification of the virus and serological analysis of the isolate showing CPE in the fourth blank transfer after inoculation of primary calf kidney cells weredone at the Institute of Virology, Veterinary Faculty of Bern University, Switzerland. Serum samples (194 in total) of the affected kid goats and mothers that had produced diseased offspring were also sent to this institution in order to identify the presence of CAEV antibodies by ELISA and immunoblotting methods. An agar gel precipitation test was also performed by the same institute to screen for Mycoplasma capricolum in the serum samples. Thirty milk samples from female goats that had produced diseased kids were analyzed for Mycoplasma capricolum by liquid and solid culturing [2]. Besides, serum Cu and zinc levels were also determined for the 40 samples that gave birth to kids with congenitally twisted carpal joints by Unicam atomic absorption spectrometry in Turkey.
Clinical signs and congenitally twisted joints were similar in the goat kids of 2~3 months of age. The curvature was generally observed in both legs and less frequently in one leg (Fig. 1). Diseased animals with the twisted joints moved with difficulty and slowly due to pain. The twisted carpal joints were swollen. In addition, one goat kid was stiff-legged. Necropsy results showed that most twisted carpal joints were swollen with excessive yellowish synovial fluid in the synovial space. Areas of pneumonia generally appeared as small grey foci in the apical areas of diaphragmatic lobes.
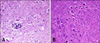
Histopathologically blood vessels in the joints were hyperemic in the subsynovial area with mononuclear cell infiltrations, mainly plasmocytes and lymphocytes, in the surrounding areas of the vessels. Synovial membrane villi were hypertrophic. Alveolar walls had thickened due to the lymphocyte and plasmocyte infiltrations. Lymphocyte clumps appeared as foci in the alveolar walls and around the capillary vessels. Hyperplasia was evident around the lymph follicles surrounding bronchioles. In the brain, eosinophil and lymphocyte infiltrations were seen in some areas of the meninges. Glial cell proliferation was also observed in the grey matter of the spinal root (Fig. 2A). Additionally, chromatolysis and pyknosis were seen in the neurons of spinal root grey matter (Fig. 2B).
All samples were negative for CAEV infection. Blood and milk samples from diseased goats and others belonging to the same herd were also negative for Mycoplasma capricolum.
The determined mean Cu levels of goats that gave birth to kids with congenitally twisted carpal joints were generally decreased (60.90 ± 2.63 µg/dL). Thirty-six serum samples had Cu levels below the normal ranges (19 to 75 µg/dL) and only two were within the limits as 124 µg/dL and 80 µg/dL, and two were undetecable. The mean zinc level was with in the normal range (142.5 ± 4.41 µg/dL).
The main finding of our field study was significantly decreased serum Cu levels indicative of a congenital Cu deficiency as previously described by Suttle and Angus [15]. Further evidence of congenital Cu deficiency was the birth of a stiff-legged goat kid during the previous year that walked on its articulus carpii. Based on the clinic pathology findings along with microbiological and biochemical results, we could diagnose the diseased animals with congenital swayback resulting from Cu deficiency rather than CAEV infection. Suttle and Angus [15] developed an experimental bovine model of Cu deficiency and found that these calves were stiff-legged. Other authors [10,14] reported arthritis in older goats but leukoencephalomyelitis were mainly seen in goat kids [11]. In addition to arthritis, interstitial pneumonia in lungs was also noted. Besides arthritis and interstitial pneumonia observed in our cases, small inflammatory foci composed of eosinophils lymphocytes were seen in the menings. Glial cell proliferation was observed in grey matter of the spinal root pyknosis and chromatholysis of the neurons were also noted in the same area. These findings were previously reported in goat kids, lambs, and calves with Cu deficiencies [3,14].
The World Health Organization categorizes Cu as an essential metal. Absence or deficiency of this element in the diet results in either functional or structural abnormalities [17]. In their study Altıntaş and Fidancı discussed the normal ranges of different biochemical markers and comparing our results to them, Cu levels of 36 out of 40 animals that born unhealthy offspring were below normal ranges (80~120 µg/dL) [1]. Also in different studies, lack of coordination, tremors, paresis, paralysis, ataxia, and muscle weakness mainly in the hind legs has been observed in the kid goats suffering from swayback caused by Cu deficiencies [6,10,12,15]. Aside from cerebral edema, cerebellar hyperplasia in gelatinous material-filled cavities, demyelination in the brain, cerebellum, and medulla spinalis and wallerian degeneration have also been reported in animals with swayback [12] but this was not seen in our study. On the contrary, we observed neural chromatholysis, pyknosis, and satellitosis in the medulla spinalis similar to that resulting from Cu deficiency as previously reported by different authors [3].
Winter et al. [16] also reported that the German Improved Fawn breed of goat suffers from a primary Cu deficiency due to insufficient mineral supplementation excluding secondary Cu deficiencies, and reported a continuous increase in serum Cu concentrations after administration of copinox to prevent ataxia [16]. We expected the animals in our study to be free of the secondary effects of Cu deficiencies. However, complex interactions between minerals that bind Cu and cause Cu deficiency, including molybdenum, sulfur, selenium, and iron, must be kept in mind. Pregnant goats may also need more than the 100 mg/day of copper, and the low birth weights may also have had an impact on serum Cu levels [4,13]. The experiment conducted by Johnson et al. [8] is particularly important as it assessed seven levels of Cu intake, including five marginally deficient Cu levels.
In conclusion, sera from goats were tested by ELISA and immunoblotting. The results indicated that the samples were negative for CAEV. Based on the pathological, microbiological, and biochemical findings, we diagnosed the animals in our study with congenital swayback resulting from Cu deficiency.
Figures and Tables
Acknowledgments
The authors would like to thank Prof. Dr. E. Peterhans from Veterinary Faculty of Bern University, Switzerland for his helpful assistance. This study was supported by the Turkish Ministry of Agriculture.
References
1. Altıntaş A, Fidancı UR. Evcil hayvanlarda ve insanda kanın biyokimyasal normal değerleri. Ankara Univ Vet Fac Derg. 1993. 40:173–l86.
2. Arısoy F, Erdağ O, Cottew GS, Watson WA. Türkiye'de koyun ve keçilerin contagious agalactia'sı üzerinde araştırmalar. Türk Vet Hekim Der Derg. 1967. 37:11–17.
3. Aupperle H, Schoon HA, Frank A. Experimental copper deficiency, chromium deficiency and additional molybdenum supplementation in goats-pathological findings. Acta Vet Scand. 2001. 42:311–321.
4. Burke JM, Miller JE. Dietary copper sulfate for control of gastrointestinal nematodes in goats. Vet Parasitol. 2008. 154:289–293.

5. Cork LC, Hadlow WJ, Gorham JR, Piper RC, Crawford TB. Pathology of viral leukoencephalomyelitis of goats. Acta Neuropathol. 1974. 29:281–292.

6. Dinev I, Petkov P, Todorov R, Kanakov D, Binev R, Petkova P. Clinical and morphologic studies of neonatal enzootic ataxia in the goat kids. II. Pathomorphologic studies. Trakia J Sci. 2005. 3:65–69.
7. Gambling L, Andersen HS, McArdle HJ. Iron and copper, and their interactions during development. Biochem Soc Trans. 2008. 36:1258–1261.

8. Johnson WT, Johnson LAK, Lukaski HC. Serum superoxide dismutase 3 (extracellular superoxide dismutase) activity is a sensitive indicator of Cu status in rats. J Nutr Biochem. 2005. 16:682–692.

9. Keen CL, Hanna LA, Lanoue L, Uriu-Adams JY, Rucker RB, Clegg MS. Developmental consequences of trace mineral deficiencies in rodents: acute and long-term effects. J Nutr. 2003. 133:5 Suppl 1. 1477S–1480S.

10. Lofstedt J, Jakowski R, Sharko P. Enzootic ataxia and caprine arthritis/encephalitis virus infection in a New England goat herd. J Am Vet Med Assoc. 1988. 193:1295–1298.
11. Narayan O, Cork LC. Lentiviral diseases of sheep and goats: chronic pneumonia leukoencephalomyelitis and arthritis. Rev Infect Dis. 1985. 7:89–98.

12. Penland JG, Prohaska JR. Abnormal motor function persists following recovery from perinatal copper deficiency in rats. J Nutr. 2004. 134:1984–1988.

13. Solaiman SG, Craig TJ Jr, Reddy G, Shoemaker CE. Effect of high levels of Cu supplement on growth performance, rumen fermentation, and immune responses in goat kids. Small Rumin Res. 2007. 69:115–123.

14. Summers BA, Appel MJG, Greisen HA, Ebel JG Jr, deLahunta A. Studies on viral leukoencephalomyelitis and swayback in goats. Cornell Vet. 1980. 70:372–390.
15. Suttle NF, Angus KW. Experimental copper deficiency in the calf. J Comp Pathol. 1976. 86:595–608.

16. Winter P, Hochsteiner W, Chizzola R. Use of copper oxide wire particles (Copinox) for the prevention of congenital copper deficiency in a herd of German Improved Fawn breed of goat. Dtsch Tierarztl Wochenschr. 2004. 111:395–397.
17. World Health Organization (WHO). Trace Elements in Human Nutrition and Human Health. 1996. WHO: Geneva;124–138.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download