Abstract
Oocytes retrieval, in vitro maturation (IVM) and fertilization (IVF) efficiency are inevitable steps towards in vitro production of embryos. In the present study, these parameters were investigated in the ovaries of prepubertal (n = 31) and pubertal (n = 61) black Bengal goats obtained from a slaughterhouse. Nuclear maturation was evaluated upon aspiration and following IVM in TCM-199 (Earle's salt with L-glutamine and sodium bicarbonate) for 27 h at 39℃ under 5% CO2 in humidified air. The oocytes retrieval and efficiency (mean ± SD) per prepubertal and pubertal goats were 5.2 ± 0.6 and 6.8 ± 0.6, and 77.3 ± 0.1% and 80.5 ± 0.6%, respectively. Anaphase I - telophase I stages differed significantly (7.3 ± 0.8 vs. 2.6 ± 0.2, p < 0.05) between the two groups of goats. After IVM, the percentages of metaphase II were significantly higher (66.3 vs. 60.3, p < 0.05) in pubertal goats than in their prepubertal counterparts. The percentages of normal in vitro fertilization (IVF) in Fert-Tyrode's albumin lactate pyruvate of pubertal goat oocytes did not differ between Percoll and swim-up sperm separation methods (36.7 ± 0.9% vs. 32.7 ± 1.3%, p > 0.05). Furthermore, sperm capacitation by heparin alone or in combination with ionomycin did not lead to a significant increase in the normal fertilization rate (34.8 ± 1.7 vs. 32.2 ± 1.5%, respectively) in the oocytes of pubertal goats. In conclusion, the ovaries of pubertal black Bengal goats obtained from the slaughterhouse could be used for in vitro embryo production. However, further optimization of the IVM and IVF techniques are necessary for satisfactory in vitro embryo production.
The preliminary goal of assisted reproductive technologies (ART) such as multiple ovulation embryo transfer and in vitro production (IVP) of embryos is to rapidly gain genetic improvement of livestock [12,28]. More recently, ART has been used for the conservation of endangered species [32], stem cell research [27] and genetic improvement. In addition, the production of large numbers of IVP embryos in an animal can increase the understanding of basic biological processes such as the endocrine control of oocyte maturation and embryo implantation or the molecular switches and metabolic pathways regulating early embryo development [12].
The IVP comprises three inevitable processes, in vitro maturation (IVM), in vitro fertilization (IVF) and in vitro culture. Maturation of mammalian oocytes is defined as the sequence of events occurring from the germinal vesicle stage to completion of the second meiotic division with formation of the first polar body [33]. The importance of oocyte quality, one of the most important intrinsic factors involved in the developmental competence of embryos, might be more appropriate to determine the oocyte's nuclear and cytoplasmic maturation, which are attained during its growth in the follicle [45]. A competent oocyte is by definition able to sustain embryonic development to term [13]. Nuclear maturation is characterized by the oocyte's ability to resume meiotic division up to metaphase II during IVM. Nuclear maturation can be visualized by the extrusion of the first polar body and the appearance of the metaphase plate using a nuclear staining technique such as Hoechst 33342 [42] or orcein [26,47]. Cytoplasmic maturation is dictated by the entire array of maternal mRNAs, proteins, substrates, nutrients and mitochondrial accumulation in the ooplasm during folliculogenesis [4,13,53]. Importantly, cytoplasmic maturation enables the oocytes to control the first cleavage divisions until the embryonic genome is activated and takes over this responsibility [9]. Follicular diameter [8,29,40], follicle status [49], oocyte diameter [8,37], cumulus morphology [56] and reproductive status of animals [49] are some factors that have been linked to the maturational competence of oocytes and have therefore been proposed as selection criteria for oocyte quality. Good quality oocytes are indeed competent for fertilization and embryo production in vitro. In the quest for good quality oocytes Wright et al. [54] showed that pubertal ewe oocytes are superior to prepubertal oocytes for fertilization both in vivo and in vitro. Similarly, pubertal oocytes were found to be of superior quality when compared to prepubertal oocytes in cows [22,29].
However, data regarding oocyte retrieval, IVM and IVF are lacking in black Bengal goats in Bangladesh. To the best of our knowledge, only Islam et al. [18] conducted a qualitative assessment of ovaries from black Bengal goats obtained in a slaughterhouse and the oocytes recovery rate. Therefore, we evaluated the oocyte retrieval rate, IVM and IVF potential of prepubertal and pubertal oocytes from black Bengal goats prior before implementing in vitro embryo production.
Different sperm preparation techniques are used in ART to separate spermatozoa with normal appearance and higher motility [5]. In cattle, it has been shown that sperm preparation techniques increased sperm motility [39] and fertilization rates [38]. Additionally, treatment of sperm with ionomycin at different concentrations increases the fertilization rate in goats [52]. Moreover, heparin is widely used for capacitation of spermatozoa in cattle IVF. This indicates the importance of capacitation agents for successful IVF in many species. Hence, in this study, we attempted to identify a suitable sperm separation method for preparation of spermatozoa and to select an agent for capacitation of spermatozoa to improve the potential for fertilization of black Bengal goats.
All chemicals, reagents, media, biologics and media constituents were purchased from Sigma-Aldrich Chemicals, USA, unless otherwise stated. All maturation and fertilization media were filtered with membrane filter (0.22 µm pore size; Millipore, Ireland) and routinely equilibrated at 39℃ under 5% CO2 in humidified air for at least 1 h prior to use.
Goat ovaries were collected at a slaughterhouse and transported to the laboratory in a thermo flask containing warm physiological saline (35℃, 0.9% sodium chloride solution, w/v) supplemented with penicillin-streptomycin (125 µg/mL Streptopen; Renata Bangladesh, Bangladesh) within 2 h of slaughtering the animal. In the laboratory, the ovaries were rinsed 3 times in physiological saline at 35℃. Follicular fluid was then aspirated from all visible follicles using an 18-gauge needle (Terumo, China) attached to a 10-mL disposable plastic syringe (Steriopack Disposable Syringe; Opso Saline, Bangladesh). The retrieved follicular fluid was then transferred to a 60 mm Petri dish (Greiner Bio-One, Germany) and diluted with TL-HEPES, after which the samples were examined for cumulus-oocyte complexes (COCs) under a stereomicroscope (MZ6; Leica Microsystems, Germany). The TL-HEPES was prepared according to Sirard et al. [44]. The COCs were washed three times in fresh TL-HEPES and once in maturation medium prior to being placed into maturation drops.
The basic medium for oocytes maturation was tissue culture medium-199 (TCM-199, Earle's salt with L-glutamine and sodium bicarbonate; Invitrogen, USA). On the day of maturation, TCM-199 was supplemented with 0.25 mM sodium pyruvate, 10% (v/v) fetal bovine serum (Invitrogen, USA), 0.05 µg/mL bovine follicle stimulating hormone (FSH; Sioux Biochemical, USA), 5 µg/mL luteinizing hormone (LH; Soiux Biochemical, USA), 1 µg/mL estradiol and 50 µg/mL gentamycin. Next, 50 µL droplets of maturation medium were prepared in a 35 mm Petri dish (Greiner Bio-One, Germany) and covered with mineral oil. Ten to fifteen oocytes were then placed in each drop and cultured in the incubator at 39℃ under 5% CO2 in humidified air for 27 h.
Frozen semen from five bucks obtained from the BRAC Bull and Buck Station (Sombugunj, Mymensingh, Bangladesh) was used for IVF. One straw (0.25 mL straw, 120 × 106 sperm / straw) from each batch was thawed at 37℃ for 30 sec. The thawed semen was layered onto a discontinuous Percoll gradient column in a 15 mL centrifuge tube (bottom layer 2 mL 90% and top layer 2 mL 45%). The tube was then centrifuged at 1,500 rpm for 10 min in a centrifuge (Centra-CL2; International Equipment, USA), and the supernatant was discarded after leaving 100~200 µL of the sperm pellet. The sperm concentration in the pellet was determined using a hemocytometer and then adjusted to 80 × 106/mL with capacitation-Tyrode's albumin lactate pyruvate (CAP- TALP) supplemented with fatty acid free BSA (6 mg/mL). The diluted semen was then mixed with CAP-TALP (1:1, v/v) containing 20 µg/mL heparin and 400 nM ionomycin (final concentrations in the IVF droplet = 10 µg/mL heparin and 200 nM ionomycin) and incubated for 15 min at 39℃ under 5% CO2 in a humidified air atmosphere.
In the swim-up method, 500 µL of semen were layered below 1 mL of CAP-TALP and then incubated at 39℃ for 60 min in a humidified air atmosphere under 5% CO2. Following incubation, 800 µL of the supernatant was recovered and centrifuged at 1,500 rpm for 10 min three times, with 700 µL of supernatant being discarded and replaced with 700 µL CAP-TALP after each round. The sperm concentration in the pellet was determined using a hemocytometer and adjusted to 80 × 106/mL with CAP-TALP. The diluted semen was then mixed with CAP-TALP (1:1, v/v) containing 20 µg/mL heparin and 400 nM ionomycin at final concentrations of 10 µg/mL and 200 nM ionomycin, respectively, after which the samples were incubated for 15 min at 39℃ under 5% CO2 in a humidified air atmosphere.
IVF-TALP was used for sperm-oocytes co-incubation. Four to five droplets (38 µL) of IVF-TALP were prepared in a 35 mm Petri dish, covered with mineral oil and allowed to equilibrate in the incubator for 1 h before sperm-oocytes co-incubation. After 27 h of maturation, the expanded COCs were removed from the IVM droplets, washed three times in washing TALP and then once in IVF-TALP. Next, five COCs in 5 µL IVF-TALP were transferred to each droplet of IVF-TALP, after which 2 µL penicillamine, hypotaurine and epinephrine (PHE; 20 µM D-penicillamine, 10 µM hypotaurine, and 1 µM epinephrine) and 5 µL capacitated sperm suspension were added to each fertilization droplet. Spermatozoa and COCs were co- incubated for 18 h at 39℃ under 5% CO2 in a humidified air atmosphere.
Upon aspiration from the follicles, oocytes were recovered and denuded using 3% sodium citrate solution [36]. Conversely, in vitro matured oocytes were placed in 1.5 mL eppendorf tubes with minimum TL-HEPES and denuded by vortexing for 3 min. One drop of TL-HEPES was then placed on separate clean dry glass slides for each group of denuded oocytes. The oocytes were then picked up (5~10 oocytes) by a mouth controlled pipette and kept in the drops. The drops were then covered with a clear glass cover slip (18 × 18 mm) after making a rail with a paraffin and Vaseline mixture. Next, the slides were submersed in acid-alcohol (acetic acid:ethanol = 1:3) overnight to fix the oocytes. On the day of evaluation, the oocytes were washed with 70% ethanol and then stained with 1% orcein for 10 min. The oocytes were subsequently cleaned with aceto-glycerol (glycerol:acetic acid:water = 1:1:3) and sealed with clear finger nail polish and examined under a differential interference contrast microscope (BX-51; Olympus, Japan).
In experiment 1, the oocytes retrieval efficiency from prepubertal and pubertal black Bengal goat ovaries was determined. The goats were classified as prepubertal or pubertal based on the absence or presence of corpus luteum, hemorrhagicum or albicans on either of the ovaries [3]. Before aspiration, all visible follicles on the ovaries of each goat were counted. Following aspiration of the follicular fluid, the number of COCs recovered from each goat was counted and the oocytes retrieval efficiency was calculated.
In experiment 2, the stages of oocyte nuclei upon aspiration (immature oocytes) with respect to prepubertal and pubertal goats were determined by nuclear staining. By observing nuclear stages, the nuclei were classified into germinal vesicle (GV), GV breakdown (GVBD), metaphase I (M I) and anaphase I-telophase I (AI-TI) stages.
In experiment 3, the metaphase II (M II) rate of oocytes was evaluated based on examination of the nuclei after staining at 27 h post-IVM. Oocytes containing M II nuclei with the 1st polar body were regarded as mature oocytes.
In experiment 4, the IVF rate of pubertal goat oocytes was evaluated by examination of the pronuclei formation after staining at 18 h post-insemination with respect to two sperm preparation procedures (Percoll separation vs. swim-up). The 18 h post-insemination oocytes were then classified as fertilized normally, fertilized by more than one spermatozoa or activated oocytes on the basis of the presence of two pronuclei, more than two pronuclei or one pronucleus, respectively.
In experiment 5, the IVF rate of the goats of pubertal oocytes were examined on the basis of two sperm capacitation methods (heparin vs. heparin + ionomycin) using techniques similar to those employed in experiment 4.
All values were expressed as the mean ± SD. Differences between parameters were evaluated by a student's unpaired t-test. All statistical analyses were conducted using SPSS (SPSS, USA). The difference between parameters was regarded as significant when the p value was less than 0.05.
The number of oocytes retrieved from each prepubertal and pubertal goat was 5.2 ± 0.6 and 6.8 ± 0.6, respectively (Table 1). Among 62 ovaries from 31 prepubertal goats, 207 follicles were aspirated and 160 COCs were retrieved, giving an oocyte retrieval efficiency of 77.3 ± 0.1%. In contrast, 517 follicles were aspirated and 416 COCs were retrieved from 122 ovaries of 61 pubertal goats, giving an oocyte retrieval efficiency of 80.5 ± 0.5%.
The results of the nuclear status of oocytes at the time of collection are presented in Table 2. The percentages of oocytes at GV stages did not different between prepubertal and pubertal goats (63.6 ± 1.6 vs. 63.9 ± 1.2). Similarly, the percentages of GVBD in the two groups did not differ significantly (23.6 ± 1.1 vs. 27.7 ± 1.0, p > 0.05). GV with a clear nuclear membrane and GVBD without a nuclear membrane are shown in Figs. 1A and B, respectively. The percentages of M I were 5.5 ± 1.0 and 5.8 ± 1.2, whereas the percentages of AI-TI were 7.3 ± 0.8 and 2.6 ± 0.2 in prepubertal and pubertal goats, respectively. The percentages of AI-TI oocytes in the two groups of goats differed significantly (p < 0.05).
The rates of IVM are presented in Table 3. The percentage of mature oocytes was 60.3 ± 2.6 and 66.3 ± 8.4 in prepubertal and pubertal goats, respectively (p < 0.05). In contrast, the percentage of oocytes in the AI-TI/MI stage in pubertal goats was higher (19.5 ± 3.1 vs. 15.5 ± 0.6) than that of the prepubertal goat group, but this difference was not significant (p > 0.05). Although no significant differences were observed in the percentage of GV (5.0 ± 1.3 vs. 6.9 ± 1.5, p > 0.05) in pubertal and prepubertal goats, a significantly lower percentage of GVBD (9.3 ± 1.9 vs. 17.2 ± 0.5, p < 0.05) was observed in the pubertal goats than in their prepubertal counterparts. An in vitro matured oocyte with a polar body and M II stage chromosome is shown in Fig. 2A.
The rates of IVF following Percoll and swim-up separation of spermatozoa are shown in the Table 4. The rates of single pronucleus formation (46.9 ± 1.5 vs. 45.5 ± 1.3%), normal fertilization with two pronuclei (36.7 ± 0.9 vs. 32.7 ± 1.3%) and polyspermic fertilization (16.3 ± 0.9% vs. 21.8 ± 0.8%) did not differ significantly between the two sperm separation methods (Percoll vs. swim-up). Normally fertilized oocytes, oocytes with one pronucleus and polyspermic fertilization are shown in Figs. 2B-D, respectively.
The effects of sperm capacitation agents on the IVF rates of oocytes derived from pubertal goats are shown in Table 5. The rates of one pronucleus formation (47.2 ± 2.9 vs. 49.4 ± 2.4%), normal fertilization (34.8 ± 1.7 vs. 32.2 ± 1.5%) and polyspermic fertilization (18.0 ± 1.5 vs. 18.4 ± 0.7%) did not differ significantly (p > 0.05) between two sperm treatment agents (heparin vs. heparin + ionomycin).
The oocyte retrieval efficiency, in vitro development ability and IVF potential must be considered before in vitro embryo production, transfer to the recipient and / or transgenesis can be conducted. In addition, reproductive status, for instance prepubertal and pubertal state of the donors, influences the efficiency of ART. This study demonstrated that more oocytes with higher IVM rates can be recovered from pubertal black Bengal goats than from their prepubertal counterparts. Due to fewer ovary and oocytes being obtained from prepubertal goats, we could not compare the fertilization rates between groups.
In this experiment, the COCs retrieval efficiency in prepubertal and pubertal goats was greater than 77% and 80%, respectively. Our oocyte recovery rate was much higher than that of other studies, which range from 33~90% [2,14,41]. The number of oocytes recovered from each group was also relatively high in the present study, being 5.2 in prepubertal and 6.8 in pubertal goats. Indeed, Islam et al. [18] only recovered 1.9 COCs from each ovary in the same breed of goat. The discrepancies in oocyte retrieval efficiency and number of oocytes recovered per goat might be due to individual variations, procedures of aspiration [22], seasonal variation [8], body weight and goats reared in the presence of bucks [50] as well as the quality of the follicles [19] present in the ovaries collected at slaughter. Judging the morphological features of oocytes is the most important criteria when selecting COCs before IVM. In this study, we assessed the morphological features of oocytes upon aspiration from the follicles and following IVM using 1% orcein. In immature oocytes, the proportion of GV was almost same in both prepubertal (63.6%) and pubertal (63.9%) goats, which indicates that these oocytes would be useful for IVM. Moreover, the lower proportion of AI-TI stage oocytes found in pubertal goat oocytes (2.6%) than prepubertal goat oocytes (7.3%) may indicate that pubertal goat oocytes would be useful for IVM. It has been shown that nuclear maturation occurs more rapidly in vitro than in vivo [24]. It is obvious that chromosome condensation starts with the occurrence of GVBD. In live cows, GVBD has been shown to occur 4~6 h after preovulatory LH peaks [25], while in superovulated cows, it has been shown to occur 6~12 h after the start of maturation [55]. Therefore, it is speculated that GV or GVBD stage immature oocytes are more competent for IVM. Because, the immature oocytes, those are already in AI or TI stage may be termed as secondary oocytes and these oocytes are proceeding to second meiotic division [15] which should be expected during IVM condition. Since, immature oocytes with multilayered compact cumulus investment showed better developmental competence in IVM and subsequent fertilization and development to the morula and blastocyst stages [7,23], pubertal goat oocytes with lower AI-TI levels might be more competent for IVM.
Based on the above findings, we proceeded with IVM to validate our results. After IVM, we found higher percentages (66.3%) of M II stage oocytes in the pubertal goat group than the prepubertal group (60.3%). Furthermore, the percentage of AI-TI/M I stage oocytes was also higher (19.5%) in pubertal than prepubertal group goats (15.5%). It is said that the presence of AI-TI stages of oocytes are also an indication of maturity toward the M II stage [10,11]. Importantly, the percentages of both GV and GVBD after IVM were higher (6.9% and 17.2%, respectively) in prepubertal goats than pubertal goats (5.0% and 9.3%, respectively). These findings indicate that the oocytes recovered from prepubertal goats are less competent for IVM. Our oocyte maturation rate (66.3%) was somewhat lower when compared with the results of other studies (71.4 to 81.4%) [1,19]. These variations might be due to breed and / or seasonal variations [8] or to the culture environment [16,17]. Furthermore, IVM of oocytes is influenced by the supplementation of gonadotrophins in TCM-199 medium. Specifically, LH alone failed to improve the oocyte maturation; however, the addition of FSH and oestradiol increased the proportion of matured oocytes [48]. In the present study, we supplemented the medium with both FSH and LH. However, gonadotrophins and other chemicals and / or reagents differ from batch to batch; therefore, additional studies should be conducted using various batches of these chemicals.
In the present study, we showed that oocytes collected from pubertal goats were more competent for IVF due to their higher IVM rate. Moreover, there are reports that oocytes from prepubertal goats show higher percentages of polyspermic fertilization and lack of sperm head decondensation up to the male pronucleus stage [36], low percentage of blastocysts formation [19] and production of high percentages of haploid embryos [51]. The sperm separation method has a great influence on IVF, cleavage and embryo development. In bovines, the Percoll separation method produced better results for selecting spermatozoa and enhanced capacity for embryo production than that of swim-up separation [6]. Somfai et al. [45] obtained a sperm population with a higher viability and acrosome integrity by Percoll separation than that of the swim-up method. In pubertal goats, Percoll density-gradient centrifugation was found to be superior to the swim-up and glass-wool methods for separating spermatozoa from frozen-thawed semen for IVF [43]. The results of the present study were in agreement with the above findings, although the difference in fertilization rate was not significant. Importantly, we found less polyspermic fertilization in the Percoll gradient sperm separation group than in the swim-up separation group. However, separation of sperm from frozen and thawed buffalo semen by the swim-up method showed better results in IVF in buffalo [34]. In this study, our normal fertilization rate was about 37%, which was somewhat lower than that observed in other studies [21,36]. To improve the fertilization rate, we added the sperm capacitating agent ionomycin to the fertilization medium along with heparin since an increased fertilization rate was observed in response to the addition of ionomycin in goats [52]. However, we did not find a significantly higher fertilization rate using the same doses of ionomycin as described by Wang et al. [52]. The decreased percentage of sperm penetration to oocytes could have been due to the use of PHE in the fertilization droplets, or to the fact that the concentrations of PHE used were not appropriate in goats [35]. In cattle, some research groups [35,46] obtained higher percentages of cleavage when PHE was present in the fertilization drops, while others [30,31] found that it had no effect. Thus, evaluating sperm for defects that cannot be compensated for in black Bengal goats such as DNA damage and sperm DNA-chromatin packaging should be conducted in future studies.
Although reports have already been published regarding the developmental potential of prepubertal goat oocytes when compared with adult goat oocytes, to the best of our knowledge, the present study of black Bengal goats is the first of its nature in Bangladesh. Due to indiscriminate breeding, the black Bengal goat has already lost its genetic merit and fertility. However, ART might enable rapid propagation of black Bengal goats within the shortest possible time. Before starting the routine use of ART, it is necessary to determine the nuclear status and IVM rate of black Bengal goat oocytes collected from slaughterhouses under Bangladesh climate conditions. The results presented here represent a first step toward the use of ART in black Bengal goats.
In conclusion, pubertal black Bengal goat ovaries obtained at a slaughter showed better rates of oocyte recovery and IVM. In addition, the Percoll gradient sperm separation technique may be more expedient than the swim-up method for IVF in pubertal black Bengal goats. However, further optimization of IVM is needed to increase the maturation rate, which, in turn, would facilitate routine use of IVM oocytes for IVF in Bangladesh.
Figures and Tables
Fig. 1
Immature oocyte. (A) Germinal vesicle stage. (B) Germinal vesicle breakdown stage. 1% orcein stain and differential interference contrast (DIC) microscopy. ×400.

Fig. 2
(A) An in vitro matured oocyte with a polar body (black arrow) and metaphase II stage chromosomes (white arrow). (B) Normally fertilized oocyte. (C) Oocytes with one pronucle. (D) Polyspermic fertilization. 1% orcein stain and DIC microscopy, ×400.
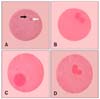
Table 2
Nuclear status of immature oocytes of black Bengal goats with respect to reproductive status

Acknowledgments
The authors thank the Mymensingh slaughterhouse authority for permitting collection of the goat ovaries. This study was supported by USDA Grant No. BG-ARS-121.
References
1. Anguita B, Jimenez-Macedo AR, Izquierdo D, Mogas T, Paramio MT. Effect of oocyte diameter on meiotic competence, embryo development, p34 (cdc2) expression and MPF activity in prepubertal goat oocytes. Theriogenology. 2007. 67:526–536.

2. Baldassarre H, Wang B, Kafidi N, Gauthier M, Neveu N, Lapointe J, Sneek L, Leduc M, Duguay F, Zhou JF, Lazaris A, Karatzas CN. Production of transgenic goats by pronuclear microinjection of in vitro produced zygotes derived from oocytes recovered by laparoscopy. Theriogenology. 2003. 59:831–839.

3. Bhuiyan MM, Suzuki Y, Watanabe H, Matsuoka K, Fujise Y, Ishikawa H, Ohsumi S, Fukui Y. Attempts at in vitro fertilization and culture of in vitro matured oocytes in sei (Balaenoptera borealis) and Bryde's (B. edeni) whales. Zygote. 2009. 17:19–28.

4. Blondin P, Coenen K, Guilbault LA, Sirard MA. In vitro production of bovine embryos: developmental competence is acquired before maturation. Theriogenology. 1997. 47:1061–1075.

5. Boomsma CM, Heineman MJ, Cohlen BJ, Farquhar C. Semen preparation techniques for intrauterine insemination. Cochrane Database Syst Rev. 2007. CD004507.

6. Cesari A, Kaiser GG, Mucci N, Mutto A, Vincenti A, Fornés MW, Alberio RH. Integrated morphophysiological assessment of two methods for sperm selection in bovine embryo production in vitro. Theriogenology. 2006. 66:1185–1193.

7. Crozet N, Ahmed-Ali M, Dubos MP. Developmental competence of goat oocytes from follicles of different size categories following maturation, fertilization and culture in vitro. J Reprod Fertil. 1995. 103:293–298.

8. Duarte G, Flores JA, Malpaux B, Delgadillo JA. Reproductive seasonality in female goats adapted to a subtropical environment persists independently of food availability. Domest Anim Endocrinol. 2008. 35:362–370.

9. Fair T, Hyttel P, Greve T. Bovine oocyte diameter in relation to maturational competence and transcriptional activity. Mol Reprod Dev. 1995. 42:437–442.

10. Fulka J Jr, Jung T, Moor RM. The fall of biological maturation promoting factor (MPF) and histone H1 kinase activity during anaphase and telophase in mouse oocytes. Mol Reprod Dev. 1992. 32:378–382.

11. Fulka J Jr, Moor RM, Fulka J. Mouse oocyte maturation: meiotic checkpoints. Exp Cell Res. 1995. 219:414–419.

12. Galli C, Duchi R, Crotti G, Turini P, Ponderato N, Colleoni S, Lagutina I, Lazzari G. Bovine embryo technologies. Theriogenology. 2003. 59:599–616.

13. Gandolfi TA, Gandolfi F. The maternal legacy to the embryo: cytoplasmic components and their effects on early development. Theriogenology. 2001. 55:1255–1276.

14. Gibbons A, Bonnet FP, Cueto MI, Catala M, Salamone DF, Gonzalez-Bulnes A. Procedure for maximizing oocyte harvest for in vitro embryo production in small ruminants. Reprod Domest Anim. 2007. 42:423–426.

15. Gordon I. Laboratory Production of Cattle Embryos. 1994. Wallingford: CAB International;30–142.
16. Han D, Lan GC, Wu YG, Han ZB, Wang HL, Tan JH. Factors affecting the efficiency and reversibility of roscovitine (ROS) block on the meiotic resumption of goat oocytes. Mol Reprod Dev. 2006. 73:238–246.

17. Han D, Zhao BT, Liu Y, Li JJ, Wu YG, Lan GC, Tan JH. Interactive effects of low temperature and roscovitine (ROS) on meiotic resumption and developmental potential of goat oocytes. Mol Reprod Dev. 2008. 75:838–846.

18. Islam MR, Khandoker MAMY, Afroz S, Rahman MGM, Khan RI. Qualitative and quantitative analysis of goat ovaries, follicles and oocytes in view of in vitro production of embryos. J Zhejiang Univ Sci B. 2007. 8:465–469.

19. Izquierdo D, Villamediana P, López-Bejar M, Paramio MT. Effect of in vitro and in vivo culture on embryo development from prepubertal goat IVM-IVF oocytes. Theriogenology. 2002. 57:1431–1441.

20. Katska-Ksiazkiewicz L, Opiela J, Ryńska B. Effects of oocyte quality, semen donor and embryo co-culture system on the efficiency of blastocyst production in goats. Theriogenology. 2007. 68:736–744.

21. Katska-Ksiazkiewicz L, Ryńska B, Gajda B, Smorag Z. Effect of donor stimulation, frozen semen and heparin treatment on the efficiency of in vitro embryo production in goats. Theriogenology. 2004. 62:576–586.

22. Keskintepe L, Darwish GM, Kenimer AT, Brackett BG. Term development of caprine embryos derived from immature oocytes in vitro. Theriogenology. 1994. 42:527–535.

23. Khurana NK, Niemann H. Effects of oocyte quality, oxygen tension, embryo density, cumulus cells and energy substrates on cleavage and morula/blastocyst formation of bovine embryos. Theriogenology. 2000. 54:741–756.

24. King WA, Bousquet D, Greve T, Goff AK. Meiosis in bovine oocytes matured in vitro and in vivo. Acta Vet Scand. 1986. 27:267–279.

25. Kruip TAM, Cran DG, van Beneden TH, Dieleman SJ. Structural changes in bovine oocytes during final maturation in vivo. Gamete Res. 1983. 8:29–47.
26. Liu RH, Sun QY, Li YH, Jiao LH, Wang WH. Maturation of porcine oocytes after cooling at the germinal vesicle stage. Zygote. 2003. 11:299–305.

27. Liu W, Yin Y, Long X, Luo Y, Jiang Y, Zhang W, Du H, Li S, Zheng Y, Li Q, Chen X, Liao B, Xiao G, Wang W, Sun X. Derivation and characterization of human embryonic stem cell lines from poor quality embryos. J Genet Genomics. 2009. 36:229–239.

28. Lohuis MM. Potential benefits of bovine embryo-manipulation technologies to genetic improvement programs. Theriogenology. 1995. 43:51–60.

29. Lonergan P, Monaghan P, Rizos D, Boland MP, Gordon I. Effect of follicle size on bovine oocyte quality and developmental competence following maturation, fertilization, and culture in vitro. Mol Reprod Dev. 1994. 37:48–53.

30. Long CR, Chase CN, Balise JJ, Duby RT, Robl JM. Effect of sperm removal time, sperm concentration and motility enhancers on fertilization parameters and development of bovine embryos in vitro. Theriogenology. 1993. 39:261.

31. Long CR, Damiani P, Pinto-Correia C, MacLean RA, Duby RT, Robl JM. Morphology and subsequent development in culture of bovine oocytes matured in vitro under various conditions of fertilization. J Reprod Fertil. 1994. 102:361–369.

32. Luvoni GC. Current progress on assisted reproduction in dogs and cats: in vitro embryo production. Reprod Nutr Dev. 2000. 40:505–512.

33. McGaughey RW. Regulation of oocyte maturation. Oxf Rev Reprod Biol. 1983. 5:106–130.
34. Mehmood A, Anwar M, Naqvi SM. Motility, acrosome integrity, membrane integrity and oocyte cleavage rate of sperm separated by swim-up or Percoll gradient method from frozen-thawed buffalo semen. Anim Reprod Sci. 2009. 111:141–148.

35. Miller GF, Gliedt DW, Rakes JM, Rorie RW. Addition of penicillamine, hypotaurine and epinephrine (PHE) or bovine oviductal epithelial cells (BOEC) alone or in combination to bovine in vitro fertilization medium increases the subsequent embryo cleavage rate. Theriogenology. 1994. 41:689–696.

36. Mogas T, Palomo MJ, Izquierdo MD, Paramio MT. Developmental capacity of in vitro matured and fertilized oocytes from prepubertal and adult goats. Theriogenology. 1997. 47:1189–1203.

37. Otoi T, Yamamoto K, Koyama N, Tachikawa S, Suzuki T. Bovine oocyte diameter in relation to developmental competence. Theriogenology. 1997. 48:769–774.

38. Parrish JJ, Krogenaes A, Susko-Parrish JL. Effect of bovine sperm separation by either swim-up or Percoll method on success of in vitro fertilization and early embryonic development. Theriogenology. 1995. 44:859–869.

39. Parrish JJ, Susko-Parrish JL, Leibfried-Rutledge ML, Critser ES, Eyestone WH, First NL. Bovine in vitro fertilization with frozen-thawed semen. Theriogenology. 1986. 25:591–600.

40. Pavlok A, Lucas-Hahn A, Niemann H. Fertilization and developmental competence of bovine oocytes derived from different categories of antral follicles. Mol Reprod Dev. 1992. 31:63–67.

41. Pierson J, Wang B, Neveu N, Sneek L, Côté F, Karatzas CN, Baldassarre H. Effects of repetition, interval between treatments and season on the results from laparoscopic ovum pick-up in goats. Reprod Fertil Dev. 2004. 16:795–799.

42. Pursel VG, Wall RJ, Rexroad CE, Hammer RE, Brinster RL. A rapid whole-mount staining procedure for nuclei of mammalian embryos. Theriogenology. 1985. 24:687–691.

43. Rho GJ, Hahnel AC, Betteridge KJ. Comparisons of oocyte maturation times and of three methods of sperm preparation for their effects on the production of goat embryos in vitro. Theriogenology. 2001. 56:503–516.

44. Sirard MA, Parrish JJ, Ware CB, Leibfried-Rutledge ML, First NL. The culture of bovine oocytes to obtain developmentally competent embryos. Biol Reprod. 1988. 39:546–552.

45. Somfai T, Bodó S, Nagy S, Papp AB, Iváncsics J, Baranyai B, Gócza E, Kovács A. Effect of swim up and Percoll treatment on viability and acrosome integrity of frozen-thawed bull spermatozoa. Reprod Domest Anim. 2002. 37:285–290.

46. Susko-Parrish JL, Wheeler MB, Ax RL, First NL, Parrish JJ. The effect of penicillamine, hypotaurine, epinephrine and sodium metabisulfite on bovine in vitro fertilization. Theriogenology. 1990. 33:333.

47. Talukder AK, Shamsuddin M, Rahman MB, Bari FY, Parrish JJ. Normal and abnormal fertilisation of zebu cattle oocytes in vitro. J Embryo Transf. 2009. 24:89–95.
48. Totey SM, Pawshe CH, Singh GP. In vitro maturation and fertilization of buffalo oocytes (Bubalus bubalis): Effects of media, hormones and sera. Theriogenology. 1993. 39:1153–1171.

49. Vassena R, Mapletoft RJ, Allodi S, Singh J, Adams GP. Morphology and developmental competence of bovine oocytes relative to follicular status. Theriogenology. 2003. 60:923–932.

50. Véliz FG, Poindron P, Malpaux B, Delgadillo JA. Positive correlation between the body weight of anestrous goats and their response to the male effect with sexually active bucks. Reprod Nutr Dev. 2006. 46:657–661.

51. Villamediana P, Vidal F, Paramio MT. Cytogenetic analysis of caprine 2- to 4-cell embryos produced in vitro. Zygote. 2001. 9:193–199.

52. Wang B, Baldassarre H, Tao T, Gauthier M, Neveu N, Zhou JF, Leduc M, Duguay F, Bilodeau AS, Lazaris A, Keefer C, Karatzas CN. Transgenic goats produced by DNA pronuclear microinjection of in vitro derived zygotes. Mol Reprod Dev. 2002. 63:437–443.

53. Watson AJ. Oocyte cytoplasmic maturation: A key mediator of oocyte and embryo developmental competence. J Anim Sci. 2007. 85:13 Suppl. E1–E3.
54. Wright RW Jr, Anderson GB, Cupps PT, Drost M, Bradford GE. In vitro culture of embryos from adult and prepuberal ewes. J Anim Sci. 1976. 42:912–917.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download