Abstract
This study describes the expression of heat shock protein70 (HSP70) and alpha-basic-crystallin (α-BC) and their association with apoptosis and some related adaptor proteins in the pathogenesis of foot-and-mouth disease virus (FMDV)-induced myocarditis in lambs. HSP70 was generally overexpressed in the myocardial tissues and inflammatory cells of FMDV-induced myocarditis with differential accumulation and localization in same hearts when compared to non-foot-and-mouth disease control hearts. α-BC immunolabeling showed coarse aggregations in the Z line of the cardiomyocytes in FMDV-infected hearts in contrast to control hearts. Overall, the results of this study show that the anti-apoptotic proteins, HSP70 and α-BC, were overexpressed with increased apoptosis in FMDV-infected heart tissues. Both proteins failed to protect the cardiomyocytes from apoptosis as defense mechanisms to the FMDV during the infection, suggesting that the virus is able to increase apoptosis via both downregulation and/or upregulation of these anti-apoptotic proteins.
Foot-and-mouth disease (FMD) is a highly contagious, severe, and clinically acute vesicular disease that affects members of the order Arteriodactyla. This order includes cloven-hoofed animals, including domesticated ruminants and pigs, as well as more than 70 wildlife species. FMD is a worldwide concern because it is enzootic in vast areas of Africa, Asia, Europe, and South America (except the Guyanas and Chile). The foot-and-mouth disease virus (FMDV) is classified within the Aphthovirus genus of the Picornaviridae family. The seven principal antigenic serotypes are the classical A, O, and C types, SAT-1, SAT-2, SAT-3, and Asia-1 [5].
FMD is characterized by vesicle-formation in the mouth and on the feet, teats, and mammary glands of older animals [5,23]. The mortality in young animals, particularly lambs and piglets, could be due to acute myocarditis, which is considered to be a fatal form of FMD that occurs without vesiculation in young animals [2,49]. Residual lesions in older cattle qualify as myocarditis with a significant inflammatory response [39]. The virus can usually be isolated from the myocardia in areas containing necrotic myocytes infiltrated with mononuclear cells or can be visualized in infected hearts by molecular techniques [18,24,40].
Heat shock proteins (HSPs) are a family of proteins that intensify their expressions in cells stressed with heat, toxins, free radicals etc. [7]. Their chaperone functions are well-recognized roles, such as folding and the transport of a variety of proteins under normal physiological conditions and following stress stimuli [7,45]. HSPs are involved in both the pro- and anti-apoptotic pathways [26,31,41]. Abnormal distribution such as translocation to the cell membrane or extracellular localization of HSPs with increased expressions of Bcl-2 and active caspase 3 is correlated with increased apoptosis [41]. In addition to these functions, the immunoregulatory functions of some HSPs have been described in the activation of the innate and adaptive immune system [4,38,46-48].
In cardiovascular biology and diseases, HSPs exhibit different protective functions, including the repair of ion channels, restoration of redox balance, interaction with the nitric oxide pathway, inhibition of proinflammatory cytokines, and inhibition of the apoptotic pathway [17,26]. HSP family members can occur at multiple points along the apoptotic pathway and be formed during the multiple steps involved in the death process and mechanisms of caspase activation [6,17]. HSP60 has been demonstrated to form a complex with pro-caspase 3 to accelerate its maturation by proteases during apoptosis [51]. Moreover, HSP27, HSP70 and HSP90 are known to display anti-apoptotic functions by inhibiting the occurrence of cytochrome c/apoptotic protease activating factor 1 complex at various points [6,11,29,36]. Alpha-basic-crystallin (α-BC) is highly abundant in cardiac muscle cells and interacts with the three major cytoskeletal components, microtubules, intermediate filaments, and microfilaments, in response to stress [8,30].
Limited studies have been conducted to investigate the pathogenetic mechanisms of myocarditis associated with FMDV in lambs [24,40]. However, the role of HSP70 and α-BC in the pathogenesis of myocarditis in FMD has not yet been studied. Therefore, this study was conducted to investigate the expressions of HSP70 and α-BC and their association with apoptosis in FMDV-infected lamb myocardium.
Tissues were obtained upon necropsy from native, approximately one- to two-week-old Karayaka lambs (n = 9) that had died during an FMD outbreak in the Samsun Province of Northern Turkey. Myocardial tissue specimens were collected from the interventricular, atrial, and ventricular portions of their hearts and then fixed in buffered formalin. The tissues were then embedded in paraffin, sectioned at 5 µm, and stained with hematoxylin and eosin. Additional sections were cut at 5 µm and mounted on organosilane (3-aminopropyl) triethoxysilane-coated glass slides for the immunohistochemical study. The remaining unfixed myocardial specimens from the infected lambs were used for virus isolation and gene sequence analysis. The studies associated with virus isolation and identification, including partial 1D gene sequence analysis and in situ reverse transcription for FMDV type O mRNA and detailed pathological examination of heart sections, were conducted in accordance with our earlier study [24].
The myocardial tissues were obtained from apparently healthy, approximately one- to two-week-old Karayaka lambs (n = 4) from a local sheep flock. The animals were euthanized under deep anesthesia according to the specifications of the Ondokuz Mayis University Animal Ethics Committee and used as controls.
The paraffin-embedded tissues were sectioned into 4 to 5 µm thick sections, placed on superfrost slides, and deparaffinized in xylene for 15 min and then in 100% ethanol for 15 min. A commercial streptavidin-biotin peroxidase complex (SABC; Zymed Laboratories, USA) was used in the preparation. All sections were pre- incubated in 10% goat non-immune serum (Zymed Laboratories, USA) at room temperature for 10 min to block the nonspecific binding of the second-step antibody (Zymed Laboratories, USA). Next, tissue sections were reacted with primary antibodies including HSP70 (1:50; Stressgen Bioreagent, Canada), α-BC (1:256; Stressgen Bioreagents, Canada), desmin (1:50; Lab Vision, USA), Bcl-2 (1:50; Diagnostic Biosystem, USA), active caspase 3 (1:100; Diagnostic Biosystem, USA), active caspase 8 (1:50; Diagnostic Biosystem, USA) and active caspase 9 (1:10; Thermo Scientific, USA) for 3 h, after which they were rinsed with PBS, pH 7.4, at room temperature (Table 1). The sections were subsequently reacted with biotin-conjugated second-step antibody (Zymed Laboratories, USA) for 10 min at room temperature, after which they were rinsed in PBS. To inactivate the endogenous peroxidase, the sections were incubated in 0.3% H2O2/methanol for 60 min. The sections were then rinsed again with PBS, after which they were reacted with SABC for 10 min at room temperature. Following another wash with PBS, the sections were incubated with 3-amino-9-ethylcarbazole (Zymed Laboratories, USA) for 15 min and then counterstained with Mayer's hematoxylin. Four negative controls were prepared, first by omitting the primary antibodies, and then by replacing them with PBS, normal rabbit serum, and unrelated mouse monoclonal antibodies.
The cellular and subcellular distributions of HSPs and apoptosis-associated proteins in heart tissues were determined and the intensity of the immunoreaction was classified as negative (-), weak (±), moderate (+), or dense (++).
To detect DNA fragmentation, the formalin-fixed, paraffin-embedded sections of myocardia were stained using the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) method (POD kit; Roche Diagnostics, Germany) according to the manufacturer's instructions. The paraffin-embedded sections were de-waxed, rehydrated, and irradiated at 350W in 0.1 µM citrate buffer, pH 6.0, in a microwave oven for 5 min. After washing twice in PBS, the sections were covered with 50 µL of the TUNEL reaction mixture containing terminal deoxynucleotidyl transferase and fluorescein-dUTP (2'-deoxyuridine 5'-triphosphate), after which they were incubated under a coverslip in a humidified chamber for 1 h at 37℃. The sections were then checked using Fluorescence microscopy. The reaction was ended by washing the slides in PBS, after which the slides were incubated with anti-fluorescein-horseradish peroxidase conjugate diluted 1:3 in 100 mM Tris-HCl, 150 mM NaCl (pH 7.5), and 1% blocking reagent for 40 min at room temperature. After washing three times for 15 min each in PBS, the sections were stained through incubation with the chromogenic substrate 3,3-diaminobenzidine for 5 to 15 min at room temperature. Sections from uninfected lambs served as controls for this procedure.
The immunohistochemical findings are summarized in Table 2. HSP70 immunolabeling was observed in the sub-endocardial cardiomyocytes of the infected hearts. Immunolabeling was diffuse and intracellular in the myocardial components, showing denser immunoreaction than the controls. Occasionally, the HSP70 was observed in a coarse granular pattern along the plasma membrane of the cardiomyocytes. However, the cytoplasm of the cardiomyocytes that was strongly labeled for HSP70 along their plasma membranes showed weak immunolabeling. A weak HSP70 signal was observed, especially in areas where inflammatory cells and severely affected cardiomyocytes were present. Perinuclear immunolabeling in some cardiomyocytes and many interstitial spindle cells showed dense immunoreaction for HSP70. Extracellular localization of HSP70 was also observed extensively in the interstitial areas (Figs. 1A-C).
α-BC immunolabeling had diffusely cytoplasmic and/or linear Z-band localization in the cardiomyocytes and was more evident in the FMDV-infected hearts than the controls. Unlike HSP70, the α-BC immunoreaction was weak in the cardiomyocyte cytoplasm, except for the striations. In severely destroyed myocardial areas, the cardiomyocytes revealed an abnormal accumulation of α-BC with coarse granules in the FMDV-infected hearts, as seen in HSP70. The strongest immunolabeling for α-BC among the myocardial tissues was observed in the Purkinje fibers in both infected and control hearts when compared with the HSP70. Surprisingly, α-BC also showed nuclear immunolabeling in some sections (Figs. 1D-F).
Desmin immunolabeling is limited in the Z-band lines of myofibers in both the control and infected hearts (Fig. 1G). In the severely affected hearts, the desmin distribution in the myofibers revealed spotty or coarse granular aggregates among the Z bands that resembled the α-BC immunolabeling (Fig. 1H). Cardiomyocyte striations in both the affected and control hearts showed strong evidence of colocalization of α-BC and desmin, possibly in the Z-band lines of the myofibers (Figs. 1D and G).
In the myocardia of the FMDV-infected lambs, the TUNEL assay indicated that the cardiac myocytes, several interstitial cells (including macrophages, lymphocytes, and fibroblasts) and a few endothelial cells had nuclei that displayed morphologic changes typical of apoptosis. However, the TUNEL-positive signal was noted in a few cardiomyocytes and interstitial cells in the myocardial sections of the control lambs (Figs. 2A and B).
Numerous cardiomyocytes, interstitial and inflammatory cells scattered throughout myocardium, both outside and adjacent to the degenerated or necrotic areas tested immunopositive for active caspase 3. Immunolabeling for caspase 3 was diffusely dispersed in the cytoplasm with occasional linear localization close to the cell membrane and nucleus of the cardiomyocytes (Figs. 2C and D). Active caspase 8 and 9 immunolabeling was detected in the cytoplasm of the cardiomyocytes in the affected areas of the infected hearts, where immunolabeling of caspase 9 was more evident than that of caspase 8 (Figs. 2E and F). Control hearts displayed a small proportion of caspase 3 in the interstitial cells (Fig. 2C), but no expression of caspases 8 or 9 was evident in any cellular components.
Control hearts showed nuclear and/or diffuse cytoplasmic labeling for Bcl-2 in a few cardiomyocytes and interstitial cells (Fig. 2G). In the infected hearts, dense Bcl-2 immunoreactivity was detected as granules in the cell and nuclear membranes, and sometimes diffusely in the cytoplasm of the cardiomyocytes, interstitial and inflammatory cells, and Purkinje fibers (Figs. 2H-J).
This study is the first report of the association of HSPs and apoptosis-related proteins in myocarditis of FMDV-infected lambs. The results of the present study clearly demonstrate that HSP70 was overexpressed in both myocardial tissues and inflammatory cells in myocarditis associated with FMD in lambs when compared with control hearts.
HSPs, which are involved in several intracellular processes, can have both pro- and anti-apoptotic actions [41]. Normally, HSPs family members might be present in different locations in the cellular compartments [20,28,50]. In this study, a decrease in the cytoplasmic expression of HSP70 against an increase along the cell membrane was observed. The cell membrane and nuclear and/or extracellular movement of HSP70 in various myocardial tissues clearly showed that abnormal accumulations and translocations were associated with increased apoptosis in FMDV-infected lambs. These findings were concordant with the results of earlier studies [20,31,41,42,50]. The loss of cellular expression of HSP70 in some areas of the affected hearts may be explained by their increased extracellular concentrations, as observed in a previous study [13,41]. In addition, it can be assumed that the loss of HSP70 expression was associated with a virus-host cell interaction. HSPs, when located in the extracellular environment or on the plasma membrane, have been found to play key roles in stimulating the immune system [34]. Extracellular and membrane bound HSPs have cytokine functions that induce the production of pro-inflammatory cytokines by the monocyte-macrophage system [47,48]. Accordingly, the extracellular localization and/or membrane movement of HSP70 observed in the present study may be indicative of the cytokine function of HSP in myocarditis.
Similarly, anti-apoptotic Bcl-2 plays an important role in several different membrane compartments and has been determined in various pathological conditions [1,10]. Overexpression of HSPs increases the amount of anti-apoptotic proteins such as Bcl-x and Bcl-2 [43]. A similar distribution of Bcl-2 and HSP70 on the outer nuclear membrane and/or cytoplasmic membranes of various myocardial elements in some areas was observed, suggesting that HSP70 increases the Bcl-2 expression in cells that play a role in FMDV pathogenesis, thus indicating shared participation of the HSPs and Bcl-2 in the apoptotic pathway [43].
Multiple proteins from picornaviruses are known to be involved in the alteration of apoptotic homeostasis of infected host cells; however, the mechanisms of cell death of the picornaviruses are unknown [12]. One of the potential mechanisms of the death of infected cells is apoptosis [12]; however, a recent in vitro study revealed that the FMDV-infected cells do not undergo apoptosis-mediated cell death [16]. We previously speculated that nitric oxide and inflammatory cells significantly influence the occurrence of myocarditis as well as the apoptosis and necrosis of cardiomyocytes in FMDV-infected lambs [24].
Overexpression of anti-apoptotic proteins HSPs and Bcl-2 is known to prevent apoptosis in several pathological conditions [15,25,52]. In this study, the loss or a low percentage of cardiomyocytes and other cellular elements expressing Bcl-2 and HSP70 were observed in some areas in which a higher percentage of TUNEL-positive elements were noted. This situation could be explained by the fact that FMDV-induced increased apoptosis with the downregulation of Bcl-2 and HSP70 expression in the myocardium. Conversely, the overexpression of both Bcl-2 and HSP70 in sections with increased numbers of TUNEL-positive cells with caspase 3 overexpression was observed. Anti- or proapoptotic function of Bcl-2 expression is associated with the Bcl-2 levels. Specifically, a low level of Bcl-2 expression is antiapoptotic, whereas a high level of expression is proapoptotic to Fas-mediated apoptosis [44]. Furthermore, the overexpression of Bcl-2 could cause increased cell death during the interaction of Bcl-xL with other factors [25]. Anti-apoptotic proteins, the members of the Bcl-2 family, are converted into potent lethal proteins when they are cleaved by caspases [15]. Grandgirard et al. [22] reported that alpha-viruses can cause apoptosis in Bcl-2 overexpressing cells by caspase-mediated proteolytic inactivation of Bcl-2. Another study demonstrated that HSP70 overexpression protects the Jurkat T cells against thermoprotection, but enhances apoptotic cell death [32]. Based on these observations, we suggest that the overexpression of anti-apoptotic proteins such as the HSP70 and Bcl-2 families failed to protect cardiomyocytes against apoptosis during FMDV infection. Indeed, FMDV may cause apoptosis in myocardial tissues via the overexpression of Bcl-2 and HSP70.
Immunohistochemical localization of α-BC in cardiomyocytes is only observed in the central portion of I bands (Z bands) where desmin is expressed [8,33]. An in vitro study revealed that α-BC plays a role in the formation of desmin filament networks in cardiac tissue [37] and effectively inhibits the aggregation of desmin filaments under various stressful conditions in situ. Further, caspase proteolysis of desmin inhibits the integrity of the intermediate filaments and actively promotes apoptosis in myogenic cells [14]. Armer et al. [3] showed that FMDV-infected cells undergo changes in the distribution of microtubule and intermediate filament components, such as F-actin, γ-tubulin and vimentin, during infection. Therefore, in the present study, desmin was selected to demonstrate the interaction along with the HSPs and cytoskeletal proteins in the cardiomyocytes. Similar distributions of α-BC and desmin in the Z line of the cardiomyocytes of infected lambs were observed, which immunolabeled both cross striations and the coarse granular aggregates among these cross-striations. Similar aggregations of the desmin filament and α-BC in the Z line of the cardiomyocytes could indicate that both proteins might cause the interactions in cardiomyocyte injury, thus demonstrating the cytoskeleton degradation of the cardiomyocytes by caspase activation during apoptosis of cardiomyocytes due to FMDV infection. These findings show that the desmin intermediate filaments influence the apoptosis of cardiomyocytes in FMD despite the α-BC overexpression.
TUNEL technique has been extensively utilized for apoptosis in tissue sections, but the interpretation and specificity of this assay are controversial [19]. To validate the TUNEL assay, the specificity of the method was tested by additional immunolabeling for enzymes and cleaved proteins considered specific for apoptosis. The results showed the widespread and similar localization of TUNEL-positive and active caspase 3 signals of various cellular elements (mainly cardiomyocytes and several interstitial cells, primarily inflammatory cells). These findings suggest that classical apoptosis occurs in a caspase-dependent manner in the myocardial damage of FMDV-infected lambs.
Active caspase 3 was detected in the cytoplasm and/or nucleus and was sometimes linearly close to the inside surface of the plasma membrane, suggesting that caspase 3 had circulated among the various cellular compartments during apoptosis. The subcellular localization of caspase 3 is most often reported in the nuclear and/or apoptotic bodies of dying cells; however, some antibodies selectively labeled caspase 3 in the cytoplasm of cells showing a morphology consistent with apoptosis, as well as in the cytoplasm of healthy-appearing cells, although occasional nuclear staining was observed [19]. The expression in the inner surface of the plasma membrane or cytoplasmic caspase 3 may suggest the early stages of apoptosis, and subsequent transferring to the nucleus [9,21]. The results encountered in some myocardial tissues also agreed with a cytological study in which the activation of caspase-3 was observed close to the cell membrane surface, after which it was transferred to the cytoplasm, and finally translocated into the nuclear region [21,27]. Further, active caspase 8 and 9 expression with caspase 3 revealed that the deaths of cardiomyocytes involved both the mitochondrion-dependent and receptor-dependent cascades of the apoptotic pathway in FMD-related myocardial damage [35].
In conclusion, the variations in the degrees of expression of the HSP70 observed in the different locations (cell membrane, nuclear and/or extracellular) in the various myocardial tissues, as well as the role of α-BC with desmin in cytoskeleton degradation of the cardiomyocytes in FMDV-infected lambs showed that HSPs play important roles in the pathogenesis of the disease. Proteins like Bcl-2 and caspase 3 involved in the apoptotic pathway could translocate among membrane-bound sites at different stages of apoptosis during FMDV infection. Although HSPs serve as a protective mechanism to increase cellular survival, the present findings indicate that their increased expressions could not protect the myocardial tissues from apoptosis mediated by both extrinsic and intrinsic cascades during the FMDV infection. Certainly, further elucidation of the role of HSPs in the pathogenesis of FMDV-induced myocarditis may provide useful information regarding the mechanism by which the virus evades the host cells as well as the virus-host interactions during infection.
Figures and Tables
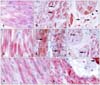 | Fig. 1(A) Diffuse moderate immunolabeling for heat shock protein70 (HSP70) in cardiomyocytes of a control heart. (B) Dense immunolabeling of some foot-and-mouth disease virus (FMDV)-infected cardiomyocytes. Note the cytoplasmic and perinuclear (arrows). (C) Granularly sarcolemmal (arrowheads) and extracellular (arrows) localization of HSP70 immunolabeling. (D) α-BC immunolabeling in cross striations of cardiomyocytes in a control heart. (E) Abnormal accumulation of α-BC with coarse granules (arrows) in severely affected cardiomyocytes in a FMDV-infected heart. (F) Perinuclear localization of α-BC in the cross section of a FMDV-infected heart (arrows). (G) Desmin immunoreactivity in cross striations of cardiomyocytes from a control heart. (H) Abnormal distribution of desmin immunolabeling with a granular pattern in a FMDV-infected heart. Arrow indicates dense immunoreaction of Purkinje fibers. Immunohistochemical staining with streptavidin-biotin peroxidase complex (SABC) and 3-amino-9-ethylcarbazole (AEC), and counterstaining with Mayer's hematoxylin. A-D: ×320, E and F: ×160, G and H: ×380. |
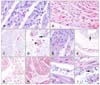 | Fig. 2(A) Few positive signals for apoptosis by the TUNEL method in the control. (B) Abundant signal in the FMDV-infected heart. (C) Caspase 3 immunolabeling in some interstitial cells of a control heart. (D) Cytoplasmic and nuclear (arrow) immunolabeling for caspase 3 of cardiomyocytes and interstitial cells in a FMDV-infected heart. Note the linear condensed immunolabeling possibly in the inner cell membrane surface (arrowhead) of the cardiomyocytes. (E) Weak cytoplasmic immunolabeling of some cardiomyocytes for caspase 8 in a FMDV-infected heart. (F) Moderate to dense immunoreaction for caspase 9 in a FMDV-infected heart. Note the presence of immunolabeling in the vessel wall (arrow). (G) Bcl-2 immunolabeling in the cellular membrane of cardiomyocytes and interstitial cells in a control heart. (H) Increased Bcl-2 immunoreaction showed various subcellular localizations in infected hearts. Note immunolabeling variations for Bcl-2. (I) High magnification of H. The nuclear and weak diffuse cytoplasmic expression in cardiomyocytes. (J) High magnification of H. Dense granular immunolabeling of cellular membrane in cardiomyocytes and interstitial cells, as well as vessels (arrow) in a FMDV-infected heart. Immunohistochemical staining with SABC and AEC, and counterstaining with Mayer's hematoxylin. A and B: ×320, C and G: ×60, D and E: ×120, F and H: ×40, I and J: ×160. |
Table 2
Cellular and subcellular distributions of HSPs and apoptosis adaptor proteins in hearts from lambs infected with foot-andmouth
disease virus (FMDV) type O

Degree of immunostaining: (-) negative, (±) weak, (+) moderate, (++) dense, CM: cardiomyocytes, IS: interstitial spindle cells, IC: inflammatory cells, VE: vascular endothelium, VS: vascular smooth muscle cells, EP: endocardium/epicardium, PF: purkinje fibers, FC: fat cells, EX: extracellular localization, CY: cytoplasmic localization, NU: nuclear localization, ME: cell membrane localization, C: control hearts, I: FMDV-infected hearts.
Acknowledgments
A part of this study was presented as a poster at the 26th Annual Meeting of the European Society of Veterinary Pathology, 17-21 September 2008, Dubrovnik, Croatia.
References
1. Akao Y, Otsuki Y, Kataoka S, Ito Y, Tsujimoto Y. Multiple subcellular localization of bcl-2: detection in nuclear outer membrane, endoplasmic reticulum membrane, and mitochondrial membranes. Cancer Res. 1994. 54:2468–2471.
2. Alexandersen S, Zhang Z, Donaldson AI, Garland AJM. The pathogenesis and diagnosis of foot-and-mouth disease. J Comp Pathol. 2003. 129:1–36.

3. Armer H, Moffat K, Wileman T, Belsham GJ, Jackson T, Duprex WP, Ryan M, Monaghan P. Foot-and-mouth disease virus, but not bovine enterovirus, targets the host cell cytoskeleton via the nonstructural protein 3Cpro. J Virol. 2008. 82:10556–10566.

4. Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000. 6:435–442.

5. Barker IK, van Dreumel AA, Palmer N. Jubb KVF, Kennedy PC, Palmer N, editors. The alimentary system. Pathology of Domestic Animals. 1993. 4th ed. San Diego: Academic Press;141–144.

6. Beere HM, Green DR. Stress management - heat shock protein-70 and the regulation of apoptosis. Trends Cell Biol. 2001. 11:6–10.

7. Benjamin IJ, McMillan DR. Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res. 1998. 83:117–132.
8. Bennardini F, Wrzosek A, Chiesi M. αB-crystallin in cardiac tissue association with actin and desmin filaments. Circ Res. 1992. 71:288–294.

9. Bressenot A, Marchal S, Bezdetnaya L, Garrier J, Guillemin F, Plénat F. Assessment of apoptosis by immunohistochemistry to active caspase-3, active caspase-7, or cleaved PARP in monolayer cells and spheroid and subcutaneous xenografts of human carcinoma. J Histochem Cytochem. 2009. 57:289–300.

10. Bruce-Keller AJ, Begley JG, Fu W, Butterfield DA, Bredesen DE, Hutchins JB, Hensley K, Mattson MP. Bcl-2 protects isolated plasma and mitochondrial membranes against lipid peroxidation induced by hydrogen peroxide and amyloid beta-peptide. J Neurochem. 1998. 70:31–39.

11. Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, Garrido C. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000. 2:645–652.

13. Calderwood SK, Mambula SS, Gray PJ Jr. Extracellular heat shock proteins in cell signaling and immunity. Ann N Y Acad Sci. 2007. 1113:28–39.

14. Chen F, Chang R, Trivedi M, Capetanaki Y, Cryns VL. Caspase proteolysis of desmin produces a dominant-negative inhibitor of intermediate filaments and promotes apoptosis. J Biol Chem. 2003. 278:6848–6853.

15. Clem RJ, Cheng EHY, Karp CL, Kirsch DG, Ueno K, Takahashi A, Kastan MB, Griffin DE, Earnshaw WC, Veliuona MA, Hardwick JM. Modulation of cell death by Bcl-xL through caspase interaction. Proc Natl Acad Sci USA. 1998. 95:554–559.
16. de los Santos T, Diaz-San Segundo F, Grubman MJ. Degradation of nuclear factor kappa B during foot-and-mouth disease virus infection. J Virol. 2007. 81:12803–12815.

17. Delogu G, Signore M, Mechelli A, Famularo G. Heat shock proteins and their role in heart injury. Curr Opin Crit Care. 2002. 8:411–416.

18. Donaldson AI, Ferris NP, Wells GA. Experimental foot-and-mouth disease in fattening pigs, sows and piglets in relation to outbreaks in the field. Vet Rec. 1984. 115:509–512.

19. Duan WR, Garner DS, Williams SD, Funckes-Shippy CL, Spath IS, Blomme EAG. Comparison of immunohistochemistry for activated caspase-3 and cleaved cytokeratin 18 with the TUNEL method for quantification of apoptosis in histological sections of PC-3 subcutaneous xenografts. J Pathol. 2003. 199:221–228.

20. Ellis S, Killender M, Anderson RL. Heat-induced alterations in the localization of HSP72 and HSP73 as measured by indirect immunohistochemistry and immunogold electron microscopy. J Histochem Cytochem. 2000. 48:321–331.

21. Feng Y, Hu J, Xie D, Qin J, Zhong Y, Li X, Xiao W, Wu J, Tao D, Zhang M, Zhu Y, Song Y, Reed E, Li QQ, Gong J. Subcellular localization of caspase-3 activation correlates with changes in apoptotic morphology in MOLT-4 leukemia cells exposed to X-ray irradiation. Int J Oncol. 2005. 27:699–704.
22. Grandgirard D, Studer E, Monney L, Belser T, Fellay I, Borner C, Michel MR. Alphaviruses induce apoptosis in Bcl-2-overexpressing cells: evidence for a caspase-mediated, proteolytic inactivation of Bcl-2. EMBO J. 1998. 17:1268–1278.

24. Gulbahar MY, Davis WC, Guvenc T, Yarim M, Parlak U, Kabak YB. Myocarditis associated with foot-and-mouth disease virus type O in lambs. Vet Pathol. 2007. 44:589–599.

25. Kim R. Unknotting the roles of Bcl-2 and Bcl-xL in cell death. Biochem Biophys Res Commun. 2005. 333:336–343.

26. Kirchhoff SR, Gupta S, Knowlton AA. Cytosolic heat shock protein 60, apoptosis, and myocardial injury. Circulation. 2002. 105:2899–2904.

27. Krajewska M, Wang HG, Krajewski S, Zapata JM, Shabaik A, Gascoyne R, Reed JC. Immunohistochemical analysis of in vivo patterns of expression of CPP32 (Caspase-3), a cell death protease. Cancer Res. 1997. 57:1605–1613.
28. Kreisel W, Hildebrandt H, Schiltz E, Köhler G, Spamer C, Dietz C, Mössner W, Heilmann C. Immuno-gold electron microscopical detection of heat shock protein 60 (hsp60) in mitochondria of rat hepatocytes and myocardiocytes. Acta Histochem. 1994. 96:51–62.

30. Launay N, Goudeau B, Kato K, Vicart P, Lilienbaum A. Cell signaling pathways to αB-crystallin following stresses of the cytoskeleton. Exp Cell Res. 2006. 312:3570–3584.

31. Lin L, Kim SC, Wang Y, Gupta S, Davis B, Simon SI, Torre-Amione G, Knowlton AA. HSP60 in heart failure: abnormal distribution and role in cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol. 2007. 293:H2238–H2247.

32. Liossis SNC, Ding XZ, Kiang JG, Tsokos GC. Overexpression of the heat shock protein 70 enhances the TCR/CD3- and Fas/Apo-1/CD95-mediated apoptotic cell death in Jurkat T cells. J Immunol. 1997. 158:5668–5675.
33. Longoni S, Lattonen S, Bullock G, Chiesi M. Cardiac alpha-crystallin. II. Intracellular localization. Mol Cell Biochem. 1990. 97:121–128.
34. Multhoff G. Heat shock protein 70 (Hsp70): membrane location, export and immunological relevance. Methods. 2007. 43:229–237.

35. Myers RK, McGavin MD. McGavin MD, Zachary JF, editors. Cellular and tissue responses to injury. Pathologic Basis of Veterinary Disease. 2007. 4th ed. St. Louis: Elsevier Mosby;3–62.
36. Pandey P, Saleh A, Nakazawa A, Kumar S, Srinivasula SM, Kumar V, Weichselbaum R, Nalin C, Alnemri ES, Kufe D, Kharbanda S. Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO J. 2000. 19:4310–4322.

37. Perng MD, Wen SF, van den IJssel P, Prescott AR, Quinlan RA. Desmin aggregate formation by R120G αB-crystallin is caused by altered filament interactions and is dependent upon network status in cells. Mol Biol Cell. 2004. 15:2335–2346.

38. Radons J, Multhoff G. Immunostimulatory functions of membrane-bound and exported heat shock protein 70. Exerc Immunol Rev. 2005. 11:17–33.
39. Robinson WF, Maxie MG. Jubb KVF, Kennedy PC, Palmer N, editors. The cardiovascular system. Pathology of Domestic Animals. 1993. 4th ed. San Diego: Academic Press;27–28.

40. Ryan E, Horsington J, Durand S, Brooks H, Alexandersen S, Brownlie J, Zhang Z. Foot-and-mouth disease virus infection in young lambs: pathogenesis and tissue tropism. Vet Microbiol. 2008. 127:258–274.

41. Sapozhnikov AM, Gusarova GA, Ponomarev ED, Telford WG. Translocation of cytoplasmic HSP70 onto the surface of EL-4 cells during apoptosis. Cell Prolif. 2002. 35:193–206.

42. Sapozhnikov AM, Ponomarev ED, Tarasenko TN, Telford WG. Spontaneous apoptosis and expression of cell surface heat-shock proteins in cultured EL-4 lymphoma cells. Cell Prolif. 1999. 32:363–378.

43. Shan YX, Liu TJ, Su HF, Samsamshariat A, Mestril R, Wang PH. Hsp10 and Hsp60 modulate Bcl-2 family and mitochondria apoptosis signaling induced by doxorubicin in cardiac muscle cells. J Mol Cell Cardiol. 2003. 35:1135–1143.

44. Shinoura N, Yoshida Y, Nishimura M, Muramatsu Y, Asai A, Kirino T, Hamada H. Expression level of Bcl-2 determines anti- or proapoptotic function. Cancer Res. 1999. 59:4119–4128.
45. Snoeckx LHEH, Cornelussen RN, Van Nieuwenhoven FA, Reneman RS, Van Der Vusse GJ. Heat shock proteins and cardiovascular pathophysiology. Physiol Rev. 2001. 81:1461–1497.

46. Srivastava PK, Amato RJ. Heat shock proteins: the 'Swiss Army Knife' vaccines against cancers and infectious agents. Vaccine. 2001. 19:2590–2597.

47. Tsan MF, Gao B. Cytokine function of heat shock proteins. Am J Physiol Cell Physiol. 2004. 286:C739–C744.

48. Tsan MF, Gao B. Heat shock protein and innate immunity. Cell Mol Immunol. 2004. 1:274–279.
49. Tunca R, Sozmen M, Erdogan H, Citil M, Uzlu E, Ozen H, Gokçe E. Determination of cardiac troponin I in the blood and heart of calves with foot-and-mouth disease. J Vet Diagn Invest. 2008. 20:598–605.

50. Welch WJ, Feramisco JR. Nuclear and nucleolar localization of the 72,000-dalton heat shock protein in heat-shocked mammalian cells. J Biol Chem. 1984. 259:4501–4513.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download