Abstract
Recently, the world's first transgenic dogs were produced by somatic cell nuclear transfer. However, cellular senescence is a major limiting factor for producing more advanced transgenic dogs. To overcome this obstacle, we rejuvenated transgenic cells using a re-cloning technique. Fibroblasts from post-mortem red fluorescent protein (RFP) dog were reconstructed with in vivo matured oocytes and transferred into 10 surrogate dogs. One puppy was produced and confirmed as a re-cloned dog. Although the puppy was lost during birth, we successfully established a rejuvenated fibroblast cell line from this animal. The cell line was found to stably express RFP and is ready for additional genetic modification.
Somatic cell nuclear transfer (SCNT) is currently the only technique suitable for producing transgenic dogs because the use of technologies appropriate for other species, such as germline-transmissible embryonic stem cells and in vitro embryo culture, have not yet been established in dogs. However, SCNT is associated with a number of limitations. To establish transgenic donor cells for SCNT, transfection and selection procedures must be performed [14]. During this process, the donor cells can easily become senescent and the number of transgenic cells that can be used for SCNT are limited [4]. In particular, gene targeting by homologous recombination or multiple transfections that require two or more rounds of selection [3] is extremely hard to perform with primary cultured cells that are necessary for SCNT.
In 1998, Cibelli et al. [3] suggested an alternative strategy to overcome these obstacles by using a serial cloning technique. In their study, they produced cloned fetuses derived from senescent bovine fibroblasts and then successfully isolated non-senescent fibroblasts from the fetuses. It had already been proven that non-senescent cells from cloned animals can be used to produce re-cloned offspring in several species including cattle [7], pigs [2], and cats [1]. Thus, the life-span of the cells can be theoretically elongated infinitely using this serial cloning technique. Furthermore, complex genetic modification could be performed as much as desired if the transgenes were successfully transferred to the re-cloned transgenic animals. Currently, the potential for serial cloning in dogs and the extent of transmission of the transgene from transgenic dogs to re-cloned dogs is unclear. Therefore, the present study was performed to produce re-cloned offspring from our red fluorescent protein (RFP) transgenic dog and to analyze expression of the RFP gene in the re-cloned dog. A re-cloned transgenic cell line for further serial cloning was also established.
In our previous report [6], four female and two male RFP dogs were successfully produced by SCNT. However, one of the male dogs was died due to chronic bronchopneumonia at 11 weeks after birth. To re-clone the deceased RFP dog (R6), we harvested fibroblasts 2 h after the death of the puppy and established a cloned transgenic cell line. The same SCNT and embryo transfer procedures described in our previous reports [6,8-10] were used for re-cloning in the present study. In total, 174 re-cloned embryos reconstructed with fibroblasts derived from R6 were transferred into the oviducts of 10 estrous-synchronized surrogate dogs. Two surrogates became pregnant but one experienced an abortion around 1 month of gestation. On Day 62 of gestation, the pregnant surrogate delivered one male puppy (rcR6). Unfortunately, the puppy was lost during birth.
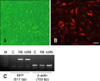
To validate that rcR6 was a clone of R6, microsatellite and mitochondrial DNA sequencing analyses were performed [11]. As shown in Table 1, rcR6 was genetically identical to the cell donor, R6, while the mitochondrial DNA sequence was identical to the oocyte donor but different from that of the R6 and surrogate dog (Table 2). These data revealed that rcR6 was a clone of R6. The expression of RFP in rcR6 was also evaluated. Similar to the RFP expression in R6 [6], rcR6 also expressed RFP in all of the examined organs (Fig. 1). Therefore, we concluded that the phenotypes of transgenic dogs can be inherited by the re-cloned offspring. To establish a re-cloned transgenic cell line, fibroblasts were harvested from rcR6 then cultured in vitro. The re-cloned fibroblasts grew robustly, were morphologically normal (Fig. 2A), and stably expressed RFP (Figs. 2B and C).
In the present study, we failed to obtain a viable re-cloned dog although the puppy almost developed to full-term and died during the delivery process. Additionally, the overall re-cloning efficiency in this report was inferior to that of a previous report on cloning R6 [6]. Thus, the canine re-cloning procedure developed in the present study still requires some improvement. Several previous reports have shown that cloning efficiency is decreased by re-cloning [5,13] especially if adult cells isolated from cloned animals are used as donor cells for SCNT [14] as was the case in the present study. However, this phenomenon can be overcome in mice by using histone deacetylase inhibitors such as trichostatin A to epigenetically reprogram donor cells, or by using stem cells derived from cloned animals as donor cells for SCNT [12]. We also found similar tendencies for both of these strategies during canine re-cloning (data not shown) and further studies are being conducted to confirm our observation.
In conclusion, we demonstrated the possibility of producing a re-cloned transgenic dog. The RFP transgene was inherited by the offspring and was highly expressed after re-cloning. A re-cloned transgenic cell line suitable for further serial cloning was also successfully established. This technology will be useful for producing dogs with multiple modified genes or gene targeting in canines.
Figures and Tables
Fig. 1
Expression of red fluorescent protein (RFP) in the organs of the re-cloned dog. (A and a) spleen, (B and b) kidney, (C and c) trachea and lung, (D and d) stomach and intestine, (E and e) liver, (F and f) heart. (A~F) Visible light images. (a~f) Fluorescence images.
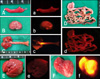
Fig. 2
Transgenic cell line established from the re-cloned dog. (A) Visible light image. (B) Fluorescence image. Scale bar = 100 µm. (C) PCR analysis of the RFP gene. M: marker, C: wild-type, R6: RFP transgenic dog, rcR6: re-cloned dog derived from R6.
Acknowledgments
This study was supported by Korea Institute of Planning & Evaluation for Technology in Food, Agriculture, Forestry & Fisheries (Grant #311011-05-1-SB010), Ministry of Knowledge and Economy (Grant #10033839-2011-13), RNL Bio, TS Corporation, Nestlé Purina PetCare, the Research Institute for Veterinary Science and the Ministry of Education, Science and Technology, through the BK21 program for Veterinary Science, Seoul National University, Korea.
References
1. Cho SJ, Bang JI, Yu XF, Lee YS, Kim JH, Jeon JT, Yee ST, Kong IK. Generation of a recloned transgenic cat expressing red fluorescence protein. Theriogenology. 2010. 73:848–855.

2. Cho SK, Kim JH, Park JY, Choi YJ, Bang JI, Hwang KC, Cho EJ, Sohn SH, Uhm SJ, Koo DB, Lee KK, Kim T, Kim JH. Serial cloning of pigs by somatic cell nuclear transfer: restoration of phenotypic normality during serial cloning. Dev Dyn. 2007. 236:3369–3382.

3. Cibelli JB, Stice SL, Golueke PJ, Kane JJ, Jerry J, Blackwell C, Ponce de León FA, Robl JM. Cloned transgenic calves produced from nonquiescent fetal fibroblasts. Science. 1998. 280:1256–1258.

4. Fujimura T, Murakami H, Kurome M, Takahagi Y, Shigehisa T, Nagashima H. Effects of recloning on the efficiency of production of α1,3-galactosyltransferase knockout pigs. J Reprod Dev. 2008. 54:58–62.

5. Hill JR, Winger QA, Long CR, Looney CR, Thompson JA, Westhusin ME. Development rates of male bovine nuclear transfer embryos derived from adult and fetal cells. Biol Reprod. 2000. 62:1135–1140.

6. Hong SG, Kim MK, Jang G, Oh HJ, Park JE, Kang JT, Koo OJ, Kim T, Kwon MS, Koo BC, Ra JC, Kim DY, Ko C, Lee BC. Generation of red fluorescent protein transgenic dogs. Genesis. 2009. 47:314–322.

7. Kubota C, Tian XC, Yang X. Serial bull cloning by somatic cell nuclear transfer. Nat Biotechnol. 2004. 22:693–694.

8. Lee BC, Kim MK, Jang G, Oh HJ, Yuda F, Kim HJ, Hossein MS, Kim JJ, Kang SK, Schatten G, Hwang WS. Dogs cloned from adult somatic cells. Nature. 2005. 436:641.

9. Oh HJ, Hong SG, Park JE, Kang JT, Kim MJ, Kim MK, Kang SK, Kim DY, Jang G, Lee BC. Improved efficiency of canine nucleus transfer using roscovitine-treated canine fibroblasts. Theriogenology. 2009. 72:461–470.

10. Oh HJ, Kim MK, Jang G, Kim HJ, Hong SG, Park JE, Park K, Park C, Sohn SH, Kim DY, Shin NS, Lee BC. Cloning endangered gray wolves (Canis lupus) from somatic cells collected postmortem. Theriogenology. 2008. 70:638–647.

11. Parker HG, Kruglyak L, Ostrander EA. Molecular genetics: DNA analysis of a putative dog clone. Nature. 2006. 440:E1–E2.
12. Van Thuan N, Kishigami S, Wakayama T. How to improve the success rate of mouse cloning technology. J Reprod Dev. 2010. 56:20–30.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download