Abstract
Infectious bovine keratoconjunctivitis (IBK) is an acute disease caused by Moraxella bovis (Mb). Several factors may predispose animals to an IBK outbreak; one commonly observed is infection with bovine herpes virus type 1 (BHV-1). The aim of this study was to investigate the dynamics of BHV-1 virus infection and its relation with clinical cases of IBK in weaned calves from a beef herd with a high prevalence of lesions caused by Mb. Sampling was carried out in six stages and included conjunctival swabs for isolating Mb as well as blood samples for identifying antibodies specific for BHV-1. A score for IBK lesions after observing each eye was determined. The findings of this study showed a high prevalence of BHV-1 virus infection (100% of animals were infected at the end of the trial); 67% of animals were culture-positive for Mb, but low rates of clinical IBK (19% of calves affected) were detected at the end of the trial. These results suggest that infection with BHV-1 did not predispose these animals to IBK, and that Mb infection produced clinical and subclinical disease in the absence of BHV-1 co-infection.
Infectious bovine keratoconjunctivitis (IBK) is an acute infectious disease caused by Moraxella bovis (Mb) [12]. This pathogen affects the cornea and ocular tissues, thereby causing severe economic losses by decreasing animal productivity and incurring costs due to treatment. IBK presents throughout the year although there are peaks in autumn and summer (due to increased ultraviolet radiation) [4] coinciding with high activity of fly populations. According to information provided by a survey of Argentinian farmers in the Province of Cordoba (Argentina) [9], this disease shows different seasonal behaviours according to the production systems. In full-cycle beef herd facilities, the highest incidence of IBK occurs in the summer while the disease is observed throughout the year in dairy and breeding herds.
Several factors may predispose the ocular structures to colonization by Mb or may even aggravate lesions produced by this infectious agent. Flies act as vectors of the agent [5] by transferring the organism from one animal to another. They are also considered to play a very important role in the epidemiology of IBK, and reported that direct transmission among animals housed in fly-free environment is minimal [5]. Vector populations increase during the summer, coinciding with increases in the number of IBK cases [1].
One of the most important infectious agents associated with IBK is the bovine herpes virus type 1 (BHV-1), which also causes of infectious bovine rhinotracheitis [11]. It was experimentally shown that this virus serves as a predisposing factor for IBK because conjunctival inoculation of pure BHV-1 cultures caused conjunctivitis and blepharitis but not keratitis, a condition which itself can be produced by inoculation with pure cultures of Mb [11]. This finding was corroborated by another assay in which calves vaccinated against BHV-1 with modified live virus vaccines were more susceptible to infection with Mb compared to unvaccinated animals [2].
In an experimental study, Pugh et al. [11] elucidated the role of BHV-1 as a risk factor for IBK. First, by inoculating calves with the virus, all animals developed viral conjunctivitis. After inoculation with Mb, 70% of animals formed IBK lesions. Second, another group of calves were first infected with Mb and then with BHV-1. Afterwards, only 50% of the animals developed IBK lesions. This led to the conclusion that previous infection by BHV-1 could act as a predisposing factor for developing clinical cases of IBK [2,11]. Based on these data and to better understand the role of BHV-1 virus in herds with endemic Mb infections, the objective of this study was to evaluate the dynamics of Mb infection and its relationship with the occurrence of IBK and BHV-1 infection in recently weaned calves from a breeding herd.
This study was conducted using 48 recently weaned Hereford × Aberdeen Angus calves 7 to 10 months of age. These animals belonging to a bovine herd of the Experiment Station of the National Institute of Agricultural Technology in Marcos Juárez, province of Córdoba, Argentina. They were maintained on a natural field grass diet supplemented with calf starter (mixed with ground corn grain, soy bean pellet, wheat bran, dicalcic phosphate, sodium chloride and a vitamin-mineral supplement), and managed in a single group. This herd was neither vaccinated against IBK nor against BHV-1, but usually presented a high number of clinical IBK cases annually. The calves received no other vaccination aside from a mandatory foot and mouth disease inactivated viral vaccine.
Calves were restrained in a hydraulic squeeze chute and both eyes were visually examined for the presence of pre-existing corneal lesions. The eyes of these animals were swabbed for bacteriological studies. Only animals negative for Mb and did not show clinical signs of IBK were included in the present study.
Once per month for 6 months, calves were gathered for sampling (S1, S2, S3, S4, S5, and S6). For each sampling, blood aliquots from individual animals were obtained. The eyes were also examined for the presence of lesions. Additionally, a sample of ocular conjunctiva was taken for bacteriological studies. All samples were obtained as described below.
Disposable syringes (5 mL; Arista, Taiwan) and needles (40 × 12; Nipro, USA) were used to extract 3 mL of blood from the jugular vein of each calf. These samples were centrifuged at 10,620 × g for 10 min at 22℃, transferred to labeled serum vials, and stored at -20℃ before subsequently detecting antibodies against BHV-1.
Samples from the conjunctiva of both eyes of each calf were taken with sterile cotton swabs. Each sample was streaked onto plates with Columbia agar (Oxoid, UK) supplemented with 5% of sterile defibrinated horse blood and incubated aerobically at 37℃ for 24 h. Next, isolation and identification of Mb were carried out according to previously described protocols [3]. Piliation was determined by observing the formation of pits in the agar and colony morphology correlated with pili expression [6]. Production of cytolisin by isolations was determined hemolysis on blood agar.
During the field trial, the presence and grade of injuries due to IBK in the calves' eyes were determined. These were classified using a numerical score as follows: 0 = normal eye, 1 = epiphora and/or conjunctivitis, 2 = corneal erosion, 3 = corneal ulceration, and 4 = internal membranes prolapsed (keratoconus) with loss of ocular substance. These data for each sampling were recorded on a chart.
To evaluate the dynamics of infection by BHV-1a, a commercial ELISA kit (IBR-Ab Test Kit; IDEXX Laboratories, USA) was used to detect the presence of anti-BHV-1a antibodies. The test was performed according to manufacturer's instructions; individual sera from each sampling were analyzed. Absorbance was measured using a 490 nm wavelength filter in a spectrophotometer (Multiskan; Lab Systems, USA) for reading 96-well plates. The absorbance values were expressed as optical density, and processed using Excel software (Microsoft, USA). A curve of concentrations antibodies anti-BHV-1 as a function of time was generated in order to observe the dynamics of immunoglobulin during the trial.
Statistical analysis was performed with an analysis of variance (ANOVA) using SPSS software (SAS, USA). The Mb isolation, lesions, and optical densities detected were compared for the six samplings. Data were then evaluated to determine whether a causal link between infection with BHV-1 and Mb could be detected. Relationships between BHV-1-positive antibody conversion and natural Mb infection were evaluated. Samples that were positive for BHV-1-specific conversion were analyzed in relation to previous Mb isolation or lesions (Kruskal-Wallis and Mann Whitney test) in order to elucidate the predisposing role of the BHV-1 virus. Differences were considered significant when p-values were less than 0.05.
Out of the 288 samples analyzed (48 animals from six samplings), 67% were bacteriologically positive for Mb at least once during the trial (Fig. 1). The number of animals from which Mb was isolated during the experiment varied for each sampling period. However, the highest number was found (p < 0.05) in the third sampling (Fig. 2A). In the subsequent samplings, these values were lower than 10% of the Mb culture-positive animals (Fig. 2A).
Out of the total number of animals with lesions, 38% of them showed some degree of ocular pathology during the experiment, but only 19% of the calves could be considered IBK+ during the study (Fig. 1). Finding lesions during sampling was not directly related to bacteriological results. As shown in Fig. 2A, the largest number of animals with lesions was found during the second sampling period, which was statistically different from the rest (p < 0.05). No differences in the incidence of clinical disease (IBK+) were observed between the sampling periods (Table 1).
For S1 and S6, no animals with eye lesions were observed (Fig. 3), while the number and diversity of lesions observed was the largest for the S2 (Fig. 3B). Most lesions observed were grade 1 (conjunctivitis with epiphora); 19% of the animals developed this type of ocular damage during the experiment. Additionally, corneal erosion (grade 2) affected 10% of the animals. Grade 3 lesions (corneal ulceration) were observed in 2% of the calves but few animals had irreversible damage. Only 2% of animals had keratoconus with ocular substance loss (grade 4) which caused permanent blindness (Fig. 3A).
Among the calves which presented lesions in one sampling, most showed signs of improvement during subsequent observations with total remission of the disease. Only 12% of the animals showed lesions for two or more subsequent sampling periods during the trial, but none were classified as grade 4. There were few blinded calves and these did not have a previous history of eye lesions, suggesting that the disease evolved within the period of two successive samplings.
Our data showed that, first, there was a peak in the number of lesions at S2 characterized mainly by conjunctivitis and keratitis, typical clinical signs of IBK. Later, the prevalence of Mb isolation reached its maximum level at S3 but fewer lesions were detected and low numbers of BHV-1-positive calves were observed. At this point, it is important to note that there was a high prevalence of Mb infection coinciding with the appearance of IBK lesions, but the percentage of animals with anti-BHV-1 antibodies was negligible. During the following observation periods (S4, S5, and S6), IBK lesions and Mb isolation rates decreased while the calves started to show evidence of BHV-1 infection. A significant increase in the percent of ELISA-positive calves was noted and reached 100% at S6 (Fig. 4).
The information presented in this paper was obtained during the growing-fattening period of beef calves undergoing an outbreak of IBK followed by infection with BHV-1. Although BHV-1 was never isolated from animals at the Estación Experimental Agropecuaria, Marcos Juárez (Argentina), this virus was observed in previous IBK studies [8] which evaluated ocular lesions such as blepharitis and/or conjunctivitis that could have been produced by infection with Mb in animals perhaps predisposed by a previous BHV-1 infection. Such pathogenic association has been studied by some researchers [2,11]. Therefore, we thought it very interesting to study the dynamics of these two agents during concomitant infection, and sought to clarify the effect of BHV-1 virus infection on the development of IBK disease in our study herd. The objective of this study was to evaluate the dynamics of Mb infection and its relationship with the occurrence of IBK and BHV-1 infection. The results have demonstrated that, at the finish of the experiment, the seroprevalence of BHV-1in the herd was 100% but only 19% of animals were IBK+.
It is known that BHV-1 is easily and directly transmitted because a large amount of virus is eliminated primarily by respiration and ocular secretions from infected animals [15]. However, the virus can also be transmitted indirectly through people or equipment [14]. Factors that were presumably important for increasing the number of infected calves in this trial may have been related to practices used to collect experimental data, the movement and concentration of animals within a small area, and immobilization with non-sanitized instruments. In fact, our data analysis showed that the animals had a low prevalence of BHV-1 infection during the first experimental stage which included the initial three sampling periods (90 days). During the subsequent sampling periods, the percentage of BHV-1-positive calves increased for some unknown causes; however, the animals did not present clinical symptoms of BHV-1 infection (respiratory or neurological symptoms). It is possible that our method of samplings favored viral spread once a few animals started to eliminate viral particles; however, we cannot explain why this phenomenon started at S4~S5.
Furthermore, our data demonstrated rapid dissemination of BHV-1 among calves given that 100% of the animals seroconverted within two samplings (S4 to S5). Nevertheless, none of the animals showed clinical signs of rhinotracheitis, possibly because of the low pathogenicity of the individual viral strain that affected this herd. Variation in herpes virulence is a known phenomenon [7] and could have influenced the low pathogenic specificity for ocular tissues in this group of calves. This corroborates the speculation that BHV-1 infection was not an important risk factor for developing IBK in our study. The increased number of animals infected with BHV-1 did not correspond to a successive increase in clinical cases of IBK despite the high level of herd Mb infection pressure.
The BHV-1 virus is considered to be an immune system depressant that inhibits the migration of polymorphonuclear neutrophils, cell-mediated cytotoxicity, the mitotic response of peripheral blood lymphocytes, and some functional activities of alveolar macrophages [15]. These viral properties might contribute to the clinical development of IBK. In addition, some animals with BHV-1 infection may also develop unilateral or bilateral conjunctivitis due to direct viral colonization of the ocular structures [15]. However, these conditions can be easily confused with keratoconjunctivitis caused by Mb and/or stimulate bacterial colonization.
During S4 to S5, we found that while 100% of the animals had been in contact with the virus as revealed by serological data, Mb isolates and lesions consistent with IBK decreased significantly. Furthermore, the low incidence of conjunctivitis or blepharitis that were detected during the period in which we estimated the virus was circulating within the herd, prior to the point of maximum serological prevalence (S4), suggested that the resulting ocular damage was minimal. Thus, BHV-1 did not seem to act as an important predisposing factor for Mb infection and/or the development of IBK lesions.
Our findings (high rates of Mb infection, 100% serological reactants to BHV-1 after Mb infection, and low rates of clinical IBK cases) did not agree with findings reported by Pugh et al. [11]. This group determined through experimental challenges that although BHV-1 is not the primary etiological agent of IBK, conjunctival or corneal infection with the virus can predispose ocular tissues to developing IBK lesions prior to infection with Mb. It must be noted that Pugh et al. [11] performed experimental inoculations while we reported results from a pasture fed herd of beef calves naturally infected with Mb and BHV-1. Under these conditions, the high prevalence of viral infection did not seem to act as an important risk factor for eye disease due to the low incidence of IBK detected after infection with the virus. Isolation of Mb from animals without IBK lesions was not surprising since it has been previously described in the literature [13]. This could be due to the great variability of Mb antigen expression [10] which would explain why particular strains sometimes result in ocular pathology while others are non-pathogenic. Antigenic variation allows infectious organisms to hide or express their antigens, and make it possible to isolate Mb bacteria from completely healthy eyes. All of our Mb isolates expressed pili and cytolisin, the main virulence factors of this bacterial species. However, the fact that the organism was also isolated from healthy eyes suggests that these are not the only factors responsible for virulence and that others are also necessary for IBK production.
These findings would allow us to infer that the BHV-1 infection does not always predispose animals to IBK clinical disease, even in the presence of Mb infection. Development of IBK might be related to other pathogenic variables rather than just to the simultaneous presence of these two agents in the same herd. Subsequent studies assessing concomitant natural and experimental infections with BHV-1 and Mb should be conducted to clarify the roles played by both agents in the development of IBK.
Figures and Tables
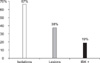 | Fig. 1Percentage of animals with isolation of Moraxella bovis (Mb), with IBK lesions and clinical IBK (IBK+). |
 | Fig. 2Relationship among the percentage of animals affected by Mb infection, IBK lesions, clinical disease. (A) Arrows indicate the maximum number of affected animals with Mb isolations or IBK lesions, and positivity for anti-infectious bovine rhinotracheitis antibody. (B) Arrow indicates when the detection of anti-BHV-1 antibodies began to increase during the experimental trial. |
 | Fig. 3Types and distribution of ocular lesions during this study. (A) Percentage of animals affected by each type of lesions. (B) Percentage of animals affected by each type of lesions during the six sampling periods. |
Acknowledgments
This work was supported by a grant from the National Agency of Science and Technology, Fondo Nacional para la promoción de la Investigación Científica y Tecnológica, Proyecto de Investigación Científica y Técnica 08 05237. We would also like to thank Prof. Flavia Bertone (English Corner Institute, Rafaela, Argentina) and Dr. Marcelo Signorini (CONICET-INTA, Rafaela, Argentina) for their collaboration with this experimental work.
References
1. Cheng TH. Frequency of pinkeye incidence in cattle in relation to face fly abundance. J Econ Entomol. 1967. 60:598–599.

2. George LW, Ardans A, Mihalyi J, Guerra MR. Enhancement of infectious bovine keratoconjunctivitis by modified-live infectious bovine rhinotracheitis virus vaccine. Am J Vet Res. 1988. 49:1800–1806.
3. Holt JG. Bergey's Manual of Systematic Bacteriology. 1994. 9th ed. Baltimore: Williams & Wilkins;296–302.
4. Kopecky KE, Pugh GW Jr, Hughes DE. Wavelength of ultraviolet radiation that enhances onset of clinical infectious bovine keratoconjunctivitis. Am J Vet Res. 1980. 41:1412–1415.
5. Kopecky KE, Pugh GW Jr, Mcdonald TJ. Infectious bovine keratoconjunctivitis: Contact transmission. Am J Vet Res. 1986. 47:622–624.
6. Lepper AWD, Hermans IR. Characterisation and quantitation of pilus antigens of Moraxella bovis by ELISA. Aust Vet J. 1986. 63:401–405.

7. Pidone CL, Galosi CM, Etcheverrigaray ME. Bovinos herpesvirus 1 y 5. Analecta Vet J. 1999. 19:40–50.
8. Piscitelli HG, Zielinski GC. Evaluación de una estrategia de control de la Queratoconjuntivitis Infecciosa Bovina. Vet Arg. 1997. 14:179–186.
9. Piscitelli HG, Zielinski GC. Encuesta epidemiológica sobre la queratoconjuntivitis infecciosa bovina en la provincia de Córdoba. Therios. 1996. 25:235–243.
10. Prieto CI, Aguilar OM, Yantorno OM. Analyses of lipopolysaccharides, outer membrane proteins and DNA fingerprints reveal intraspecies diversity in Moraxella bovis isolated in Argentina. Vet Microbiol. 1999. 70:213–223.

11. Pugh GW Jr, Hughes DE, Packer RA. Bovine infectious keratoconjunctivitis: Interactions of Moraxella bovis and infectious bovine rhinotracheitis virus. Am J Vet Res. 1970. 31:653–662.
12. Pugh GW Jr, Hughes DE, McDonald TJ. The isolation and characterization of Moraxella bovis. Am J Vet Res. 1966. 27:957–962.
13. Pugh GW Jr, McDonald TJ. Identification of bovine carriers of Moraxella bovis by comparative cultural examinations of ocular and nasal secretions. Am J Vet Res. 1986. 47:2343–2345.
14. Thielscher HH, Huth FW. IBR/IPV: Impfen oder Tilgen? Landbauforschung Völkenrode. 1986. 36:121–126.
15. Wyler RM, Engels M, Schwyzer M. Wittmann G, editor. Infectious bovine rhinotracheitis/vulvo-vaginitis. Herpesvirus Diseases of Cattle, Horses, and Pigs. 1989. Boston: Kluwer Academic;1–72.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download