Abstract
Leptin is an adipocytokine that regulates body weight, and maintains energy homeostasis by promoting reduced food intake and increasing energy expenditure. Leptin expression and secretion is regulated by various factors including hormones and fatty acids. Butyrate is a short-chain fatty acid that acts as source of energy in humans. We determined whether this fatty acid can play a role in leptin expression in fully differentiated human adipocytes. Mature differentiated adipocytes were incubated with or without increasing concentrations of butyrate. RNA was extracted and leptin mRNA expression was examined by Northern blot analysis. Moreover, the cells were incubated with regulators that may affect signals which may alter leptin expression and analyzed with Northern blotting. Butyrate stimulated leptin expression, and stimulated mitogen activated protein kinase (MAPK) and phospho-CREB signaling in a time-dependent manner. Prior treatment of the cells with signal transduction inhibitors as pertusis toxin, Gi protein antagonist, PD98059 (a MAPK inhibitor), and wortmannin (a PI3K inhibitor) abolished leptin mRNA expression. These results suggest that butyrate can regulate leptin expression in humans at the transcriptional level. This is accomplished by: 1) Gi protein-coupled receptors specific for short-chain fatty acids, and 2) MAPK and phosphatidylinositol-3-kinase (PI3K) signaling pathways.
Leptin is16-kDa non-glycosylated circulating protein hormone. It is encoded by the ob gene and produced mainly by adipocyte. Leptin is a multifunctional hormone that regulates body weight, energy homeostasis, neuroendocrine function, fertility, immune function, and angiogenesis, inflammation, and hematopoiesis [1,5,10,19,25]. Leptin carries out its biological actions on target tissues through interaction with its specific receptor (Ob-R). This receptor is a member of the gp130 family of cytokine receptors [21]. Ob-R has several variants (Ob-Ra through Ob-Rf) that are produced by alternative splicing of the db leptin receptor gene [9]. The predominant long isoform of leptin receptors (Ob-Rb) is the predominant, function and responsible for leptin actions [2]. Ob-Rb can activate the signal transducers and activators of transcription pathways. Ob-Rb and the short isoform (Ob-Ra) can transduce signals through insulin receptor substrates and through mitogen activated protein kinase (MAPK) dependent pathways [20].
Obesity is a chronic disease that concerns over a billion of adult people all over the world [1,15]. This condition involves several factors and increases the risk of diseases like metabolic syndrome, insulin resistance, type 2 diabetes mellitus and coronary heart disease [14,15]. Regulation of leptin gene expression is a highly complex process which involves multiple mediators, the relative importance of which is, as yet, undetermined. The important regulatory factors are glucocorticoids, insulin, and thyroid hormones [1]. Thyroid hormones inhibit leptin gene expression while sex steroids, such as estrogen, increase leptin mRNA levels [16]. 3T3L1 cells studies showed that treatment with propionate elevates leptin mRNA expression through G-protein coupled receptor 43 (GPCR43) [6,23].
Butyrate is a short-chain fatty acid produced in the colonic lumen by bacterial fermentation of carbohydrates and dietary fibers [22]. In the proximal large bowel, butyrate is the preferred respiratory fuel in the intestine process by β-oxidation [4]. Butyrate stimulates pancreatic secretion in humans [7]. The purpose of the present study was to assess the role of butyrate in the regulation of leptin gene expression in human adipocytes. We also explored possible signaling pathways that may be involved in this regulatory role.
Preadipocyte growth media, adipocyte differentiation medium, and poietics human preadipocytes were purchased from Cambrex Bio Science Walkersville (USA). Bovine serum albumin, bovine insulin, isobutylmethylxanthine (IBMX), and sodium butyrate were from Sigma-Aldrich Fine Chemicals (USA). Fetal calf serum (FCS) was from Trace Scientific (Australia). Dexamethasone and indomethacin were purchased from Wako Pure Chemicals (Japan). Pertussis toxin (PTX), and PD98059 (a MAPK inhibitor) were from List Biological Laboratories (USA). Wortmannin (WT; a PI3K inhibitor) was from Biomol Research Laboratories (USA). Polyclonal antibodies against phospho-p44/p42 (ERK1/2), MAPK (Thr202/Tyr204), total p44/p42 (total ERK), phospho-CREB, and total CREB were from Cell Signaling Technology (USA).
Poietics human preadipocytes were differentiated were differentiated according to the manufacturer's instructions. Briefly, when the cells reached confluency (referred to as day 0) in preadipocyte growth media containing 10% FCS, 100 U/mL penicillin and 100 µg/mL streptomycin, the cells were cultured in adipocyte differentiation medium containing antibiotics plus 10 µg/mL insulin, 1 µM dexamethasone, 200 µM indomethacin and 500 µM IBMX. The adipocytes differentiation medium was changed every 2 days to promote cell differentiation. The degree of differentiation was recorded by lipids accumulation in the cells by oil red O staining. At the end of the differentiation period (18 days), the cells were incubated with or without butyrate at a dose of 0.5, 1.0 or 5.0 mM for 48 h. RNA was extracted using TRIzol reagent (Gibco BRL, USA) and 20 µg RNA was analyzed by northern blot analysis. In a series of experiments, the cells were incubated with either butyrate (1 mM) or inhibitors as PTX (100 ng/mL), an inhibitor of Gi/Go proteins, a MAPK inhibitor, and PD98059 (10 µM) and WT in a dose of 100 nM for a specific period of time. The cells were subjected to Western blot analysis or Northern blot analysis after 48 h incubation.
Cultured preadipocytes that had reached confluence (day 0) and had undergone adipogenic differentiation at 10 and 18 days were fixed with 10% formalin in isotonic phosphate buffer for 1 h. The cells were then stained with 0.5% oil red O (Sigma-Aldrich, USA) in 60% isopropyl alcohol for 1 h and rinsed extensively with water. Lipid droplets were stained red and visualized with light microscopy.
Following the experimental treatments, cells were washed with ice-cold PBS and scraped into ice-cold lysis buffer [50 mM HEPES (pH 7.5), 150 mM NaCl, 5 mM EDTA, 20 mM sodium fluoride, 10 mM sodium pyrophosphate, 2 mM sodium vanadate, 1% Nonidet-P40, and protease inhibitor cocktail (Boehringer Mannheim, Germany)]. Harvested cells were incubated on ice for 30 min followed by centrifugation at 12,000× g for 20 min at 4℃ to obtain the cell lysate. Proteins in the cell lysate (20 µg of protein) were resolved by SDS-PAGE (10% gel) under reducing conditions and electro-blotted onto a PVDF membrane (Immobilon; Millipore, USA). The membrane was blocked for 2 h at room temperature in 5% (w/v) nonfat milk with 20 mM Tris/HCl (pH 7.5), 0.15 M NaCl, and 0.01% Tween 20. The membrane was then incubated overnight at 4℃ with primary antibodies against phosphorylated ERK1/2, total ERK, phospho CREB, and total CREB (1 : 1,000 dilutions) as an internal standard. The membrane was washed three times with 20 mM Tris/HCl (pH 7.5), 0.15 M NaCl, and 0.01% Tween 20, and incubated with horseradish peroxidase-conjugated secondary goat anti-rabbit IgG antibody, (1 : 2,000; Zymed Laboratories, USA) for 1 h at room temperature. Antibody binding was visualized using an enhanced chemiluminescence detection system (Amersham Biosciences, USA) according to the manufacture's instructions. Intensities of the immunoreactive bands were densitometrically analyzed using NIH Image program (NIH, USA).
RNA in a reverse transcription mix was amplified to measure leptin and G3PDH expression with the followings primers: to amplify a 383 bp fragment to be used as a probe for leptin mRNA, a 5'-AGTGCCTATCCAGAAAG-3', forward primer and a 5;-TGCTCAAAGCCACCACC-3' as reverse primer. For G3PDH, a 5'-ACCACTGTCCACGCCATCAC-3 as forward primer and 5'-TCCACCACCCTGTTTGCTGTA-3' as reverse primer to amplify a 453-bp fragment to be used as a probe. The leptin and G3PDH mRNA amplified by RT-PCR from total RNA recovered from bovine subcutaneous adipose tissue and subcloned into pGEM-T Easy vector (Promega, USA). The nucleotide sequence of each cDNA were confirmed and the cDNAs were used as a probes for Northern boltting. Total RNA (20 µg) was resolved on 1% agarose-formaldehyde gel, transferred onto a nylon membrane (Hybond-N+; Amersham Pharmaceutical Biotec, UK) and cross-linked under UV light for 2 min. Both prehybridization and hybridization were performed at 65℃ for 2 h and overnight, respectively, in a buffer containing 7% SDS, 0.5 M Church's phosphate buffer pH 7.2, 1 mM EDTA, and 0.5 mg/mL salmon sperm DNA (Wako Pure Chemicals, Japan). After prehybridization, the membrane was sequentially hybridized with a cDNA probe encoding human leptin and G3PDH as internal loading control. The probe was labeled with [α-32P] dCTP using Megaprime DNA labeling systems (Amersham Biosciences, USA) according to instructions provided. After hybridization, the membrane was stringently washed for 20 min twice with 2× SSC and 0.1% SDS, and once with 0.1× SSC and 0.1% SDS at 65℃ before being exposed to a phospho-imaging plate overnight. Detection and quantification of the hybridization signals were carried out using a phospho-image analyzer (BAS 2500; Fujifilm, Japan). After imaging analysis, the membranes were re-probed for G3PDH.
To confirm the maturation of human preadipocytes into mature adipocytes, oil red O staining was performed during cell differentiation at 0, 10, and 18 days. As shown in Fig. 1, the cells had a fibroblast shape from day 0 until day 10 of differentiation. After that, the cells became round and moderate amounts of lipid droplets accumulated in the cytoplasm. By day 18, the cells had matured and significant lipid droplets were seen under the microscope.
To test effect of butyrate on leptin expression, the cells were incubated with increasing concentrations of butyrate. Northern blot analysis showed that butyrate induced leptin expression at low physiological doses (0.5 and 1 mM) as seen in Fig. 2. However, higher dose of butyrate (5 mM) inhibited leptin mRNA expression in three independent experiments.
To evaluate the involvement of MAPK and phospho-CREB pathways in the butyrate signaling, the cells were treated without (0 time point) or with butyrate (1 mM) for the indicated time point up to 3 h. As seen in Fig. 3A, butyrate stimulated the expression of phospho-ERK1/2 after 5 min; this peaked at 30 min and returned to basal expression levels after 2 h. Moreover, butyrate (1 mM) induced the phosphorylation of cAMP response element binding protein (phospho-CREB) at 2 and 3 h in the differentiated human adipocytes (Fig. 3B).
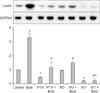
To investigate the signaling mechanism(s) underlying the effects of butyrate on leptin expression, differentiated human adipocytes were treated with butyrate (1 mM) alone or with PTX (100 ng/mL) to inactivate Gi/Go proteins. The addition of butyrate (Fig. 4) induced leptin mRNA expression. PTX alone and together with butyrate inhibited leptin expression. These same effects were observed when the cells were incubated with PD98059 (10 µM) and WT (100 nM) for 48 h, thereby confirming the involvement of GPCR, MAPK, and PI3K in the regulation of leptin expression.
The present study showed that leptin expression in human adipocytes is affected by short chain fatty acids that include butyrate. Butyrate exerts a variety of biological actions such as stimulation of exocrine and endocrine pancreatic secretions, satiety, motility of the gut and blood vessels, and proliferation of gastrointestinal tract epithelium [7]. Moreover, butyrate is well known to have in vivo and in vitro actions on endocrine and exocrine secretory functions in various species [11,12]. Intravenous administration or in vitro culturing pancreatic cells with butyrate increased the secretion of insulin, glucagon, and amylase in small ruminants [12]. Butyrate also suppresses GH expression and secretion in the ruminant in vitro and in vivo [11].
We found that butyrate up-regulated leptin expression within physiological levels (1 mM) as reported by Soliman et al. [18], but the high doses inhibited leptin expression. It is unclear why high doses of butyrate exert this inhibitory effect. Other studies have reported that butyrate inhibits cellular proliferation and induces apoptosis by regulating the key proteins which control the cell cycle [4,7]. In particular, NaB was shown to down-regulate 25 genes in colonic epithelial cells including cyclin D1, a key regulator of the G1/S phase, and the proliferating cell nuclear antigen PCNA [19]. These findings indicate that this factor possesses pro-apoptotic properties. In the study by Yonekura et al. [24], butyrate was found to stimulate leptin expression in bovine adipocytes but inhibits its expression in rat anterior pituitary cells. These observation partially concur with our previous findings in bovines [17,18,24] but not in rats, thus suggesting that the effects of butyrate are cell-specific [8,13]. Therefore, differences in butyrate-induced leptin responses between bovines, humans, and rats may be due to species-associated differences.
Butyrate induced leptin mRNA expression in a dose-dependent manner within a physiologically-relevant range of concentrations. The effective doses of butyrate in human adipocytes are comparable with those of bovine adipocytes [18], mouse adipocytes, and GPR41-transfected cells [23]. These findings partly resemble the effects of propionate on murine cells [6]. The stimulatory effect of butyrate on leptin expression and its activation of p44/p42 MPPK were inhibited by pre-treatment with PTX, an inhibitor of Gi/Go proteins [3]. Thus, we can speculate that butyrate might act on the cells through G protein-coupled receptors specific for SCFA (GPR41). Butyrate also induced phosphorylation and activation of MAPK and CREB, a downstream signal of protein kinase A activation, in a time-dependent manner. This observation confirmed the involvement of GPCRs, MAPK, and PI3K kinases signaling pathways in the regulation of leptin expression. In conclusion, the results of present study demonstrate that butyrate is an important factor which can regulate leptin gene expression in human adipocytes through GPR, MAPK, and PI3K signaling pathways.
Figures and Tables
Fig. 1
Differentiation of human preadipocytes. (A) On day 0, the cells had a fibroblast-like shape without lipid accumulation. (B) On day 10, the cells were slightly rounded and a moderate increase in lipid content was seen. (C) On day 18, the cells were more rounded and an increased number of lipid droplets were seen in the cytoplasm. Oil red O stain, ×400.
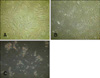
Fig. 2
Effect of butyrate on leptin mRNA expression in differentiated human adipocytes. Confluent human preadipocytes were cultured in adipocyte differentiation medium for 18 days. Values represent the mean ± SE of three independent experiments. *p < 0.05 vs. the control and #p < 0.05 vs. butyrate treatment (0.5 and 1.0 mM).
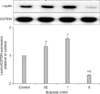
Fig. 3
Effects of butyrate on the phosphorylation of ERK1/2 (A) and phospho-CREB (B) in differentiated human adipocytes as determined by Western blotting.

Fig. 4
Leptin expression induced through different signaling pathways. Human adipocytes were cultured in the presence or absence of butyrate (Buty; 1 mM) alone or with inhibitors of signaling as (PTX; 100 ng/mL), PD98059 (PD; 10 µM), and Wortmannin (WT; 100 nM) for 48 h. Values represent the mean ± SE of three independent experiments. *p <0.05 vs. the control and #p < 0.05 vs. butyrate doses alone.
References
2. Bennett BD, Solar GP, Yuan JQ, Mathias J, Thomas GR, Matthews W. A role for leptin and its cognate receptor in hematopoiesis. Curr Biol. 1996. 6:1170–1180.

3. Blaukat A, Barac A, Cross MJ, Offermanns S, Dikic I. G protein-coupled receptor-mediated mitogen-activated protein kinase activation through cooperation of Gαq and Gαi signals. Mol Cell Biol. 2000. 20:6837–6848.

4. Coradini D, Pellizzaro C, Marimpietri D, Abolafio G, Daidone MG. Sodium butyrate modulates cell cycle-related proteins in HT29 human colonic adenocarcinoma cells. Cell Prolif. 2000. 33:139–146.

5. Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000. 68:437–446.
6. Hong YH, Nishimura Y, Hishikawa D, Tsuzuki H, Miyahara H, Gotoh C, Choi KC, Feng DD, Chen C, Lee HG, Katoh K, Roh SG, Sasaki S. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology. 2005. 146:5092–5099.

7. Katoh K, Tsuda T. Effects of acetylcholine and short-chain fatty acids on acinar cells of the exocrine pancreas in sheep. J Physiol. 1984. 356:479–489.

8. Lazar MA. Sodium butyrate selectively alters thyroid hormone receptor gene expression in GH3 cells. J Biol Chem. 1990. 265:17474–17477.

9. Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996. 379:632–635.

10. Masuzaki H, Ogawa Y, Sagawa N, Hosoda K, Matsumoto T, Mise H, Nishimura H, Yoshimasa Y, Tanaka I, Mori T, Nakao K. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat Med. 1997. 3:1029–1033.

11. Matsunaga N, Nam KT, Kuhara T, Oda S, Ohneda A, Sasaki Y. Inhibition of GH releasing factor (GRF)-induced GH secretion by intraruminal infusion of volatile fatty acids (VFA) in sheep. Endocr J. 1997. 44:133–140.

12. Matsunaga N, Nam KT, Kuhara T, Oda S, Ohneda A, Sasaki Y. Inhibitory effect of volatile fatty acids on GRF-induced GH secretion in sheep. Endocr J. 1993. 40:529–537.

13. Mitsuhashi T, Uchimura H, Takaku F. n-Butyrate increases the level of thyroid hormone nuclear receptor in non-pituitary cultured cells. J Biol Chem. 1987. 262:3993–3999.

14. Pudel V, Ellrott T. Social and political aspects of adiposis. Chirurg. 2005. 76:639–646.
15. Reincke M, Beuschlein F, Slawik M. Obesity 2006. MMW Fortschr Med. 2006. 148:20–24.
16. Saad MF, Damani S, Gingerich RL, Riad-Gabriel MG, Khan A, Boyadjian R, Jinagouda SD, el-Tawil K, Rude RK, Kamdar V. Sexual dimorphism in plasma leptin concentration. J Clin Endocrinol Metab. 1997. 82:579–584.
17. Schwartz MW, Baskin DG, Kaiyala KJ, Woods SC. Model for the regulation of energy balance and adiposity by the central nervous system. Am J Clin Nutr. 1999. 69:584–596.

18. Soliman M, Kimura K, Ahmed M, Yamaji D, Matsushita Y, Okamatsu-Ogura Y, Makondo K, Saito M. Inverse regulation of leptin mRNA expression by short- and long-chain fatty acids in cultured bovine adipocytes. Domest Anim Endocrinol. 2007. 33:400–409.

19. Tabuchi Y, Arai Y, Kondo T, Takeguchi N, Asano S. Identification of genes responsive to sodium butyrate in colonic epithelial cells. Biochem Biophys Res Commun. 2002. 293:1287–1294.

21. Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995. 83:1263–1271.

22. Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001. 81:1031–1064.

23. Xiong Y, Miyamoto N, Shibata K, Valasek MA, Motoike T, Kedzierski RM, Yanagisawa M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci USA. 2004. 101:1045–1050.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download