Abstract
Conventional lung cancer therapies are associated with poor survival rates; therefore, new approaches such as gene therapy are required for treating cancer. Gene therapies for treating lung cancer patients can involve several approaches. Among these, aerosol gene delivery is a potentially more effective approach. In this study, Akt1 kinase-deficient (KD) and wild-type (WT) Akt1 were delivered to the lungs of CMV-LucR-cMyc-IRES-LucF dual reporter mice through a nose only inhalation system using glucosylated polyethylenimine and naphthalene was administrated to the mice via intraperitoneal injection. Aerosol delivery of Akt1 WT and naphthalene treatment increased protein levels of downstream substrates of Akt signaling pathway while aerosol delivery of Akt1 KD did not. Our results showed that naphthalene affected extracellular signal-regulated kinase (ERK) protein levels, ERK-related signaling, and induced Clara cell injury. However, Clara cell injury induced by naphthalene was considerably attenuated in mice exposed to Akt1 KD. Furthermore, a dual luciferase activity assay showed that aerosol delivery of Akt1 WT and naphthalene treatment enhanced cap-dependent protein translation, while reduced cap-dependent protein translation was observed after delivering Akt1 KD. These studies demonstrated that our aerosol delivery is compatible for in vivo gene delivery.
The ability to selectively deliver transgenes to the lung will facilitate the development of gene therapies for a variety of lung diseases including cancer. The aerosol delivery of genes represents a noninvasive means of targeting the lung periphery and possibly preventing some of the problems associated with intravenous delivery and intratracheal installation [13,14,43]. Viral and non-viral vectors are currently utilized for gene therapy. Viral vectors are mainly used because of their powerful cellular infection potential. Nevertheless, many factors can limit the use of viral vectors. Viral vectors need to be inactivated in order to prevent uncontrolled proliferation of the vector or an immune response against viral proteins. Therefore, non-viral vectors, such as liposomes and polycations, have been proposed as an alternative. Among these, glucosylated polyethylenimine (GPEI) has been proven to be a promising cationic polymeric delivery vector that can transfect cells in vitro as well as in vivo [18,26,41]. GPEI was reported to increase the hydrophilicity and gene transfer efficiency while having low toxicity compared to unsubstituted polyethylenimine (PEI) [8,18]. Therefore, we used GPEI as an aerosol delivery carrier for the current study.
Akt is an oncogene transduced by an acute transforming retrovirus (Akt-8) initially isolated from an AKR thymoma cell line [40] and subsequently found to encode a serine/threonine protein kinase [2]. Akt is also known as protein kinase B. This factor has a broad range of downstream targets that regulate tumor-associated cell processes such as cell growth, cell cycle progression, survival, migration, and angiogenesis [4]. The Akt pathway is an exciting novel target for molecular therapeutics as it acts as a cardinal nodal point for transducing extracellular and intracellular oncogenic signals. Alterations of this pathway have been identified in a number of human malignancies [27]. Dominant negative alleles of Akt were reported to reduce cell survival and induce an apoptotic response [9,22]. Akt elevates both cell survival and proliferation rates. Therefore, specific inhibition of its downstream signaling pathway, for example through the expression of an Akt kinase-deficient (KD) mutant, can regulate other related signaling pathways mediated by Akt and may represent a reasonable therapeutic approach for treating tumors with elevated levels of Akt.
Most protein translation involves assembly of the eukaryotic initiation factor (eIF) 4F translation initiation complex on the 5' cap structure. This is followed by recruitment of ribosomal subunits and their associated factors. The extracellular signal-regulated kinase (ERK)-MAPK and phosphatidylinositol 3-kinase (PI3K)-Akt pathways have been shown to play key roles in regulating protein translation efficiency [3,38,47]. One mechanism by which Akt and ERK are known to alter cell function is through the regulation of cap-dependent translation [34,37]. To measure the ratio of cap-dependent to cap-independent translation in this study, we used CMV-LucR-cMyc-IRES-LucF dual reporter mice. These transgenic mice express a bicistronic vector and can be used to measure cap-dependent versus cap-independent protein translation [6,41]. Using these animals, we show that aerosol delivery of Akt1 WT or KD using GPEI can alter Akt- and ERK-related signaling pathways along with protein translation in the lungs of naphthalene-treated mice. This may provide a target for treating lung disease.
Bioactivated xenobiotic naphthalene is extensively used for synthesizing a variety of compounds such as dyes, plastics, and grinding wheels, and is a pervasive environmental contaminant. It has been reported that humans are exposed to naphthalene during its production or usage, and by smoking cigarettes [7]. Parenteral administration of naphthalene was found to cause cytotoxity in the olfactory epithelium of rats and mice [31,48]. Non-ciliated or Clara cells in the distal bronchiolar epithelium are particularly susceptible to naphthalene injury in mice [31]. Naphthalene has been shown to reduce cell death and to affect ERK [7]. The ERK signaling cascade regulates proliferation, differentiation, and survival in multicellular organisms [16]. However, the molecular mechanism through which naphthalene affects protein translation and the ERK-Akt pathway is not well understood. Here we investigated whether ERK and Akt signaling pathways are involved in naphthalene-induced Clara cell injury. We were interested in determining whether we could attenuate Clara cell injury by altering ERK- and Akt-related signaling pathway in vivo through aerosol delivery of Akt1. Therefore, we evaluated the potential effects of Akt1 WT and Akt1 KD on protein signaling pathways and Clara cell injury in the lungs of naphthalene-treated mice. Our results clearly revealed that Clara cell injury induced by naphthalene was considerably attenuated in mice exposed to Akt1 KD. We also report that naphthalene can regulate ERK protein levels, downstream effectors of ERK, and cap-dependent protein translation.
Naphthalene was purchased from Sigma-Aldrich (USA). Anti-p70S6K, anti-ERK, anti-eIF4E, anti-phospho-eIF4E, and anti-phospho-p70S6K antibodies were obtained from Santa Cruz Biotechnology (USA). Anti-CC10 antibody was obtained from Abcam (USA). WT Akt1 and mutant Akt1, in which Lys179 in the kinase domain was replaced by aspartate (K179A), were introduced into pCMV5. These constructs were generously provided by Dr. B. A. Hemmings (Friedrich Miescher Institute, Switzerland). They are referred to as Akt1 WT and Akt1 KD, respectively [41].
Six-week-old transgenic male mice (four mice/group) expressing the CMV-LucR-cMyc-IRES-LucF reporter gene [6,41] were used for this study. The mice were kept in our animal facility and maintained in a 12 h light/dark cycle at 23 ± 2℃ with a relative humidity of 50 ± 20%, and given food and water ad libitum. For each experiment, the mice were divided into five groups: an untreated control group, two groups that were exposed to an aerosol containing Akt1 WT/KD, or vector, and other two groups that were exposed to Akt1 WT/KD and or naphthalene only. All animal experiments were performed according to the guideline for the care and use of laboratory of Seoul National University, Korea.
GPEI was prepared by reacting cellobiose with PEI using cyanoborohydride as described previously [18,29]. The mice were placed in a nose-only exposure chamber (Dusturbo, Korea) and exposed to aerosol generated with a patented nebulizer (Korean patent no. 20304964) containing the GPEI/DNA complex solution for 30 min. GPEI/DNA complexes contained 1 mg Akt1 WT/KD, or pCMV5 (vector control) and 2.67 mg of GPEI. In brief, 1 mg of DNA was mixed drop by drop with 2.67 mg of carrier in a final volume of 50 mL with slow vortexing. The solution was then incubated for 30 min at room temperature prior to aerosol delivery [41]. Two days after exposure to the aerosolized GPEI/DNA complexes, the mice were sacrificed and samples of the lung were recovered.
Naphthalene was dissolved in corn oil and administrated to mice via i.p. injection. Mice received 200 mg of naphthalene/kg body weight; control animals received a comparable volume (10 mL/kg body weight) of corn oil alone. Mice that received aerosolized Akt1 complexes received naphthalene 1 day after gene delivery.
Mouse lung samples were homogenized and then digested in three volume lysis buffer (Promega, USA). Total protein concentration was quantified with Bio-Rad Protein Assay reagent (Bio-Rad, USA) before the samples underwent electrophoresis. Equal amounts of protein extracts (50 µg) were loaded onto an SDS polyacrylamide gel. After electrophoresis, the separated proteins were transferred to nitrocellulose membranes (Amersham Pharmacia, UK). The membranes were blocked with 5% skimmed milk for 1 h at room temperature and then incubated with specific antibodies diluted 1 : 1,000 (v : v) in 5% skim milk for 3 h at room temperature. After washing three times with Tris-buffered saline, 0.1% Tween 20 (TTBS), the membranes were incubated for 1 h with horseradish peroxidase-labeled secondary antibody (Zymed, USA) at room temperature. Antibody binding was visualized with a Westzol enhanced chemiluminescence detection kit (Intron, Korea). The bands were detected with an LAS-3000 imaging system (Fujifilm, Japan). For luciferase activity assay, Renilla and firefly luciferase activities were measured using the Dual-Luciferase kit (Promega, USA) according to manufacturer's instructions as previously described [41].
Lungs were removed from the dual luciferase mice. The left lobes were fixed in 10% neutral phosphate-buffered formalin overnight and embedded in paraffin. The paraffin-embedded tissues were cut (5 µm) and transferred to plus slides (Fisher Scientific, USA). For histological analysis, the tissue sections were deparaffinized in xylene, rehydrated through alcohol gradients and washed with distilled deionized water then stained with hematoxylin and eosin for light microscopy examination. For immunohistochemical analysis, the slides were immersed in xylene, rehydrated in ethanol, and washed with water. To quench endogenous peroxidase activity, the slides were immersed in 3% H2O2 for 10 min. After rinsing in phosphate-buffered saline (PBS), non-specific binding was blocked by incubating the sections in 3% bovine serum albumin (BSA) for 1 h at room temperature. After washing with PBS, the sections were incubated with the appropriate primary (1 : 200 dilution in BSA) antibodies for 3 h at room temperature, and secondary HRP-conjugated antibodies (1 : 50 dilution; Zymed, USA) for 1 h at room temperature. After washing with PBS, the sections were incubated for 5 to 10 min at room temperature with 3,3'-diaminobenzidinetetrahydrochloride substrate (Biosesang, Korea) and then counterstained with 1% Mayer's hematoxylin (Dako, USA).
Results are expressed as the mean ± SE of three experiments. Statistical analyses were performed with Student's t-test for experiments with two groups. A p-value < 0.05 was considered to be statistically significant. Quantification of the Western blot bands was performed using the Multi Gauge program (ver 2.02; Fujifilm, Japan).
Our result showed that aerosol delivery of Akt1 WT increased protein expression of p70S6K, p-p70S6K, ERK 1/2 (Fig. 1A), p-eIF4E, and eIF4E (Fig. 2A). We also observed increased expression of these proteins in the lungs of mice exposed to both Akt1 WT and naphthalene. No increases in eIF4E protein levels were observed in mice treated with naphthalene alone. However, naphthalene increased the protein levels of p70S6K, ERK 1/2, and p-eIF4E. This compound also significantly changed the protein expression of p-p70S6K. Densitometric analysis clearly confirmed the results of Western blotting analysis (Figs. 1B and 2B). Immunohistochemistry analysis confirmed that the levels of ERK (Fig. 3), p-eIF4E (Fig. 4), and p-p70S6K (Fig. 5) were elevated in treated mice compared to the control animals. These results showed patterns similar to those observed in the Western blot analysis.
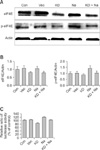
To further evaluate the effects of Akt and naphthalene on cap-dependent and -independent protein translation, dual luciferase activities were measured. Our results clearly showed that aerosol delivery of Akt1 WT and naphthalene treatment increased the relative ratio of renilla/firefly activities (Fig. 2C). Based on these findings, we further analyzed the effects of aerosol delivery of Akt1 KD and naphthalene on the Akt and ERK signaling pathways. Western blot analysis indicated that aerosol delivery of Akt1 KD did not increase the protein levels of p70S6K, p-p70S6K, ERK 1/2 (Fig. 6A), p-eIF4E, and eIF4E (Fig. 7A). However, expression of p70S6K, p-p70S6K, ERK 1/2, and p-eIF4E were all increased in mice exposed to naphthalene compared to the control. In contrast, decreased expression of these proteins was observed in mice exposed to both Akt1 KD and naphthalene compared to the mice exposed to naphthalene (Figs. 6A and 7A). Densitometric analysis clearly confirmed the results of Western blot analysis (Figs. 6B and 7B). In addition, measurement of luciferase activities showed that aerosol delivery of Akt1 KD decreased the relative ratio of renilla/firefly activities while naphthalene increased this relative ratio (Fig. 7C).
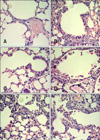
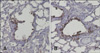
To observe histopathological changes in the lungs of mice, histopathology examination was carried out. In the mice exposed to Akt1 WT, Akt1 KD, and the vector control, no histopathological changes were observed in the bronchiolar epithelial cells (Figs. 8A~C). Histopatholgy examination revealed vacuolated or exfoliating Clara cells in the lumina of terminal bronchioles in mice exposed to naphthalene only (Fig. 8F). In mice exposed to both Akt1 WT and naphthalene, Clara cells exfoliating into the lumina of the bronchioles were observed while the remaining cells were squamated (Fig. 8D). Mice exposed to both Akt1 KD and naphthalene had Clara cells exfoliating into the lumina of the bronchioles but the Clara cell injury induced by naphthalene was considerably attenuated compared to mice exposed to naphthalene only (Fig. 8E). Our histopathological results were further confirmed by CC10 immunohistochemical staining. CC10 was used as a marker of lung injury and epithelial integrity (Fig. 9). Clara cells in the lungs of mice exposed to both Akt1 KD and naphthalene were recovered compared to the group with naphthalene alone.
Understanding the molecular basis of numerous human diseases has facilitated the development of gene-based therapies. Gene therapy is defined as treating diseases by delivering therapeutic genetic material into specific cells to beneficially alter the expression of genes which are influencing the disease process. Viral vectors are associated with high transfection efficiency but their clinical application is limited by low DNA packaging capacities, difficulties with ensuring patient safety, risk of inducing an immune response, and risk of insertional mutagenesis [5,30,52]. Recently, difficulties associated with clinical tests using viral vectors have renewed interest in these vectors. On other hand, non-viral vectors such as peptides, liposomes, and polymers have various advantages over viral vectors such as low toxicity, ease of synthesis and low immune response. Some polymeric vectors such as GPEI have been found to have the highest transfection efficiency among non-viral gene delivery vectors, which can be comparable to that of viral vectors [12,15,20,25,42]. We have previously used GPEI, a cationic polymer, as an effective delivery vehicle for gene. Our group also has demonstrated the efficacy of aerosol gene delivery using GPEI by showing that aerosol delivery of Akt using GPEI could control Akt pathway and protein translation in the lungs of dual luciferase mice [41]. Based on those promising results, we chose to use GPEI as a carrier for the present study.
Molecular genetic studies have led to the identification of different potential targets for therapeutic modalities, such as Akt [23]. The Akt signaling pathway has been shown to regulate cell proliferation and survival, and is closely associated with the progression of different lung diseases [23,33]. Elevated Akt activity has been observed in malignant human bronchial epithelial and Non-small-cell lung carcinoma (NSCLC) cells [23,24,33,39]. Therefore, targeting this pathway may be extremely useful in clinical settings, especially for treating NSCLC and other lung diseases in which constitutive activation of Akt frequently occurs. In the present study, we showed that the Akt1 KD construct could regulate Akt-related signaling pathway.
Airway injury and repair are involved in the pathogenesis of several lung diseases such as asthma, chronic obstructive pulmonary disease, and lung cancer. Among the different factors that cause lung injury, hazardous air pollutants exert chronic adverse effects on lung functions, and are reported to be partly responsible for morbidity and mortality in humans [10,21]. Clara cells, the non-ciliated cells in the epithelial lining of bronchioles, have several roles in the lungs. One of the main functions of Clara cells is to protect the bronchiolar epithelium. Naphthalene (200 mg/kg) has been reported to cause Clara cell injury [31,51]. This compound is used as feedstock for variety of chemical industrial processes, and was found to be the most plentiful polycyclic aromatic hydrocarbon in cigarette smoke that selectively injures Clara cells in the conducting airways [10,21,32,46,50]. Clara cell injury patterns observed in the present study were similar to ones previously described [31,51]. We also showed that the Clara cell injury induced by naphthalene was diminished in the lungs of mice exposed to Akt1 KD as demonstrated by histopthological and CC10 immunohistochemical analyses.
Naphthalene has been reported to decrease cell death and induce an anti-apoptotic response. This chemical induces IL-8 production that has been reported to affect ERK [7]. ERK is a part of the MAPK family of serine/threonine kinases. It is activated by phosphorylation of a tyrosine-threonine motif and in turn phosphorylates a number of downstream substrates including a consensus proline-directed serine or threonine site. The ERK signaling cascade is involved in cellular proliferation, differentiation, and survival, and its inappropriate activity is a common feature of human cancers [16,28]. Thus, regulating the ERK cascade by introducing Akt KD may be good strategy for controlling protein translation and attenuating Clara cell injury induced by naphthalene exposure. The findings of our study indicated that naphthalene affected the downstream effectors of ERK, suggesting a role for ERK-related signaling pathways in Clara cell injury.
Essential steps in protein translational control take place at the level of eIF4F and p70 S6 kinase. eIF4F is a complex whose activity involves the recognition of the RNA 5' cap structure and eukaryotic translation initiation factor. eIF4E has been shown to contribute to the formation eIF4F complex. p70 S6 kinases phosphorylate the 40S ribosomal subunit protein S6 and activate the translation of mRNA containing 5' ligopyrimidine tracts. The ERK pathway is involved in regulating this process by activating the eIF4E and p70 S6 kinases [1,11,17,44]. The results of our study clearly showed that naphthalene increased ERK, p70S6K, p-p70S6K, and p-eIF4E protein levels. Increased expression of p-p70S6K and p-eIF4E were also observed in the lungs of mice exposed to both Akt1 WT and naphthalene, while Akt1 KD decreased the phosphorylation of p70S6K and eIF4E induced by naphthalene. These finding suggested that the signaling pathway induced by naphthalene can be regulated by aerosol delivery of Akt1.
eIF4E is a target of PI3K/Akt and ERK signaling, and may behave as a point of convergence for these two pathways. PI3K signaling leads to elevated eIF4E activity following release from the 4E-BPs while ERK increases the phosphorylation of eIF4E [45,47,49]. eIF4E influences cancer-relevant processes such as apoptosis and senescence [33,34], and behaves as an oncogene alone or in combination with c-myc. Not surprisingly, these functions enable eIF4E to control protein translation although other eIF4E-associated activities may also be required [19,35,36,46].
In this study, we found that elevated ERK expression induced by naphthalene could partially activate p70S6K and eIF4E, resulting in an increase of cap-dependent protein translation and damage to Clara cell. Introduction of Akt1 WT by aerosol delivery further increased cap-dependent protein translation while Akt1 KD decreased this protein translation, thereby reducing Clara cell injury. These results indicate the involvement of Akt- and ERK-related pathways in Clara cell injury induced by naphthalene, and show that alterations in ERK-related signaling pathway caused by Akt1 KD introduction can attenuate Clara cell injury induced by naphthalene.
Figures and Tables
Fig. 1
Effects of Akt1 wild type (WT) and naphthalene on p70S6K, p-p70S6K, and extracellular signal-regulated kinase (ERK)1/2 protein expression. (A) Western blot and (B) densitometric analysis of p-p70S6K, p70S6K, and ERK1/2 expression. Con: control, Vec: vector, Akt1: Akt1 WT was delivered to the mice, Na: mice that received naphthalene alone, Akt + Na: mice exposed to both Akt1 WT and naphthalene. Data are presented as the mean ± SE (n = 3). *p < 0.05 indicating a significant difference compared to the corresponding control.
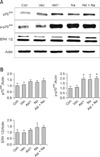
Fig. 2
Effects of Akt1 WT and naphthalene on eukaryotic initiation factor (eIF)4E and p-eIF4E protein expression. (A) Western blot and (B) densitometric analysis of eIF4E and p-eIF4E expression. (C) Effects of Akt1 on pulmonary luciferase activity in the dual reporter mice. Con: control, Vec: vector, Akt: Akt1 WT was delivered to the mice, Na: mice that received naphthalene only, Akt + Na: mice that were exposed to both Akt1 WT and naphthalene. Data are presented as the mean ± SE (n = 3). *p < 0.05 indicating a significant difference compared to the corresponding control.
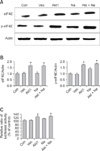
Fig. 3
Immunohistochemical staining of the lungs with anti-ERK antibody. (A) Control, (B) mice exposed to both Akt1 WT and naphthalene, and (C) mice exposed to naphthalene only. ×200.

Fig. 4
Immunohistochemical staining of lungs with anti-p-eIF4E antibody. (A) Control, (B) mice exposed to both Akt1 WT and naphthalene, (C) mice exposed to naphthalene only, and (D) mice exposed to Akt1 WT. ×200.

Fig. 5
Immunohistochemical staining of lungs with anti-p-p70S6K antibody. (A) Control, (B) mice exposed to both Akt1 WT and naphthalene, (C) mice exposed to naphthalene only, and (D) mice exposed to Akt1 WT. ×200.

Fig. 6
Effects of the Akt1 KD mutant and naphthalene on p70S6K, p-p70S6K, and ERK1/2 protein expression. (A) Western blot and (B) densitometric analysis of p-pS6K, p70S6K, and ERK1/2 expression. Con: control, Vec: vector, KD: Akt1 KD was delivered to the mice, Na: mice that received naphthalene only, KD + Na: mice that were exposed to both Akt1 KD and naphthalene. Data are expressed as the mean ± SE (n = 3). *p < 0.05 indicating a significant difference compared to the corresponding control.
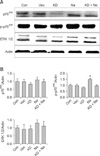
Fig. 7
Effects of Akt1 KD and naphthalene on eIF4E and p-eIF4E protein expression. (A) Western blot and (B) densitometric analysis of eIF4E and p-eIF4E expression in the lung of the luciferase mice. (C) Effects of Akt1 on pulmonary luciferase activity in the dual reporter mice. Con: control, Vec: vector, KD: Akt1 KD was delivered to the mice, Na: mice that received naphthalene only, KD + Na: mice that were exposed to both Akt1 KD and naphthalene. Data are expressed as the mean ± SE (n = 3).
Acknowledgments
This work was partly supported by the R&D Program of MKE/KEIT (10035333, Development of anti-cancer therapeutic agent based on regulating cell cycle or cell death) as well as by the National Research Foundation (NRF-2011-0000380), Ministry of Education, Science and Technology (MEST) in Korea. H. L. Jiang was supported by the Brain Korea 21 Program for Veterinary Science of Seoul National University. M. H. Cho was also partially supported by the Research Institute for Veterinary Science, Seoul National University.
References
1. Avruch J, Belham C, Weng Q, Hara K, Yonezawa K. The p70 S6 kinase integrates nutrient and growth signals to control translational capacity. Prog Mol Subcell Biol. 2001. 26:115–154.

2. Bellacosa A, Testa JR, Staal SP, Tsichlis PN. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991. 254:274–277.

3. Chang RC, Yu MS, Lai CSW. Significance of molecular signaling for protein translation control in neurodegenerative diseases. Neurosignals. 2006-2007. 15:249–258.

4. Cheng JQ, Lindsley CW, Cheng GZ, Yang H, Nicosia SV. The Akt/PKB pathway: molecular target for cancer drug discovery. Oncogene. 2005. 24:7482–7492.

6. Créancier L, Mercier P, Prats AC, Morello D. c-myc Internal ribosome entry site activity is developmentally controlled and subjected to a strong translational repression in adult transgenic mice. Mol Cell Biol. 2001. 21:1833–1840.

7. Diodovich C, Malerba I, Bowe G, Acquati F, Bianchi MG, Taramelli R, Parent-Massin D, Gribaldo L. Naphthalene exposure: effects on gene expression and proliferation in human cord blood cells. J Biochem Mol Toxicol. 2003. 17:286–294.

8. Dolivet G, Merlin JL, Barberi-Heyob M, Ramacci C, Erbacher P, Parache RM, Behr JP, Guillemin F. In vivo growth inhibitory effect of iterative wild-type p53 gene transfer in human head and neck carcinoma xenografts using glucosylated polyethylenimine nonviral vector. Cancer Gene Ther. 2002. 9:708–714.

9. Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997. 275:661–665.

10. Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, McConnell R, Kuenzli N, Lurmann F, Rappaport E, Margolis H, Bates D, Peters J. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004. 351:1057–1067.

11. Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem. 1999. 68:913–963.

12. Godbey WT, Wu KK, Hirasaki GJ, Mikos AG. Improved packing of poly(ethylenimine)/DNA complexes increases transfection efficiency. Gene Ther. 1999. 6:1380–1388.

13. Goula D, Becker N, Lemkine GF, Normandie P, Rodrigues J, Mantero S, Levi G, Demeneix BA. Rapid crossing of the pulmonary endothelial barrier by polyethylenimine/DNA complexes. Gene Ther. 2000. 7:499–504.

14. Griesenbach U, Chonn A, Cassady R, Hannam V, Ackerley C, Post M, Tanswell AK, Olek K, O'Brodovich H, Tsui L-C. Comparison between intratracheal and intravenous administration of liposome-DNA complexes for cystic fibrosis lung gene therapy. Gene Ther. 1998. 5:181–188.

15. Hwang SK, Jin H, Kwon JT, Chang SH, Kim TH, Cho CS, Lee KH, Young MR, Colburn NH, Beck GR Jr, Yang HS, Cho MH. Aerosol-delivered programmed cell death 4 enhanced apoptosis, controlled cell cycle and suppressed AP-1 activity in the lungs of AP-1 luciferase reporter mice. Gene Ther. 2007. 14:1353–1361.

16. Javle MM, Gibbs JF, Iwata KK, Pak Y, Rutledge P, Yu J, Black JD, Tan D, Khoury T. Epithelial-mesenchymal transition (EMT) and activated extracellular signal-regulated kinase (p-Erk) in surgically resected pancreatic cancer. Ann Surg Oncol. 2007. 14:3527–3533.

17. Jefferies HBJ, Fumagalli S, Dennis PB, Reinhard C, Pearson RB, Thomas G. Rapamycin suppresses 5'-TOP mRNA translation through inhibition of p70S6k. EMBO J. 1997. 16:3693–3704.

18. Kim HW, Park IK, Cho CS, Lee KH, Beck GR Jr, Colburn NH, Cho MH. Aerosol delivery of glucosylated polyethylenimine/phosphatase and tensin homologue deleted on chromosome 10 complex suppresses Akt downstream pathways in the lung of K-ras null mice. Cancer Res. 2004. 64:7971–7976.

19. Lazaris-Karatzas A, Montine KS, Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5' cap. Nature. 1990. 345:544–547.

20. Lee Y, Cho MY, Mo H, Nam K, Koo H, Jin G, Park JS. Enhancement of the transfection efficiency of poly (ethylenimine) by guanidylation. Bull Korean Chem Soc. 2008. 29:666–668.
21. Leikauf GD. Hazardous air pollutants and asthma. Environ Health Perspect. 2002. 110:Suppl 4. 505–526.

22. Li J, Simpson L, Takahashi M, Miliaresis C, Myers MP, Tonks N, Parsons R. The PTEN/MMAC1 tumor suppressor induces cell death that is rescued by the AKT/protein kinase B oncogene. Cancer Res. 1998. 58:5667–5672.
23. Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003. 4:257–262.
24. Massion PP, Taflan PM, Shyr Y, Rahman SM, Yildiz P, Shakthour B, Edgerton ME, Ninan M, Andersen JJ, Gonzalez AL. Early involvement of the phosphatidylinositol 3-kinase/Akt pathway in lung cancer progression. Am J Respir Crit Care Med. 2004. 170:1088–1094.

25. Merdan T, Kopeček J, Kissel T. Prospects for cationic polymers in gene and oligonucleotide therapy against cancer. Adv Drug Deliv Rev. 2002. 54:715–758.

26. Merlin JL, Dolivet G, Dubessy C, Festor E, Parache RM, Verneuil L, Erbacher P, Behr JP, Guillemin F. Improvement of nonviral p53 gene transfer in human carcinoma cells using glucosylated polyethylenimine derivatives. Cancer Gene Ther. 2001. 8:203–210.

27. Mitsiades CS, Mitsiades N, Koutsilieris M. The Akt pathway: molecular targets for anti-cancer drug development. Curr Cancer Drug Targets. 2004. 4:235–256.

28. Monick MM, Powers LS, Barrett CW, Hinde S, Ashare A, Groskreutz DJ, Nyunoya T, Coleman M, Spitz DR, Hunninghake GW. Constitutive ERK MAPK activity regulates macrophage ATP production and mitochondrial integrity. J Immunol. 2008. 180:7485–7496.

29. Park IK, Cook SE, Kim YK, Kim HW, Cho MH, Jeong HJ, Kim EM, Nah JW, Bom HS, Cho CS. Glucosylated polyethylenimine as a tumor-targeting gene carrier. Arch Pharm Res. 2005. 28:1302–1310.

30. Pfeifer A, Verma IM. Gene therapy: promises and problems. Annu Rev Genomics Hum Genet. 2001. 2:177–211.

31. Plopper CG, Macklin J, Nishio SJ, Hyde DM, Buckpitt AR. Relationship of cytochrome P-450 activity to Clara cell cytotoxicity. III. Morphometric comparison of changes in the epithelial populations of terminal bronchioles and lobar bronchi in mice, hamsters, and rats after parenteral administration of naphthalene. Lab Invest. 1992. 67:553–565.
32. Puchelle E, Zahm JM, Tournier JM, Coraux C. Airway epithelial repair, regeneration, and remodeling after injury in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006. 3:726–733.

33. Qian J, Zou Y, Rahman JSM, Lu B, Massion PP. Synergy between phosphatidylinositol 3-kinase/Akt pathway and Bcl-xL in the control of apoptosis in adenocarcinoma cells of the lung. Mol Cancer Ther. 2009. 8:101–109.

34. Roux PP, Shahbazian D, Vu H, Holz MK, Cohen MS, Taunton J, Sonenberg N, Blenis J. RAS/ERK Signaling Promotes Site-specific Ribosomal Protein S6 Phosphorylation via RSK and Stimulates Cap-dependent Translation. J Biol Chem. 2007. 282:14056–14064.

35. Ruggero D, Montanaro L, Ma L, Xu W, Londei P, Cordon-Cardo C, Pandolfi PP. The translation factor eIF-4E promotes tumor formation and cooperates with c-Myc in lymphomagenesis. Nat Med. 2004. 10:484–486.

38. Scheper GC, Proud CG. Does phosphorylation of the cap-binding protein eIF4E play a role in translation initiation? Eur J Biochem. 2002. 269:5350–5359.

39. Shah A, Swain WA, Richardson D, Edwards J, Stewart DJ, Richardson CM, Swinson DEB, Patel D, Jones JL, O'Byrne KJ. Phospho-Akt expression is associated with a favorable outcome in non-small cell lung cancer. Clin Cancer Res. 2005. 11:2930–2936.

40. Staal SP. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci USA. 1987. 84:5034–5037.

41. Tehrani AM, Hwang SK, Kim TH, Cho CS, Hua J, Nah WS, Kwon JT, Kim JS, Chang SH, Yu KN, Park SJ, Bhandari DR, Lee KH, An GH, Beck GR Jr, Cho MH. Aerosol delivery of Akt controls protein translation in the lungs of dual luciferase reporter mice. Gene Ther. 2007. 14:451–458.

42. Templeton NS, Lasic DD. New directions in liposome gene delivery. Mol Biotechnol. 1999. 11:175–180.

43. Uduehi AN, Stammberger U, Kubisa B, Gugger M, Buehler TA, Schmid RA. Effects of linear polyethylenimine and polyethylenimine/DNA on lung function after airway instillation to rat lungs. Mol Ther. 2001. 4:52–57.

44. Wang L, Proud CG. Ras/Erk signaling is essential for activation of protein synthesis by Gq protein-coupled receptor agonists in adult cardiomyocytes. Circ Res. 2002. 91:821–829.

45. Waskiewicz AJ, Flynn A, Proud CG, Cooper JA. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 1997. 16:1909–1920.

46. Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, Cordon-Cardo C, Pelletier J, Lowe SW. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004. 428:332–337.

47. Wendel HG, Silva RL, Malina A, Mills JR, Zhu H, Ueda T, Watanabe-Fukunaga R, Fukunaga R, Teruya-Feldstein J, Pelletier J, Lowe SW. Dissecting eIF4E action in tumorigenesis. Genes Dev. 2007. 21:3232–3237.

48. West JA, Buckpitt AR, Plopper CG. Elevated airway GSH resynthesis confers protection to Clara cells from naphthalene injury in mice made tolerant by repeated exposures. J Pharmacol Exp Ther. 2000. 294:516–523.
49. West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, Harris C, Belinsky S, Dennis PA. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003. 111:81–90.

50. Witschi H, Espiritu I, Maronpot RR, Pinkerton KE, Jones AD. The carcinogenic potential of the gas phase of environmental tobacco smoke. Carcinogenesis. 1997. 18:2035–2042.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download