Abstract
The levels of tartrate resistant acid phosphatase (TRAP), matrix metalloproteinase-2 (MMP-2), and tissue inhibitor of matrix metalloproteinase-2 (TIMP-2) in synovial fluid (SF) and serum in cases of canine osteoarthritis (OA) were measured. OA was induced by a surgically-created medial patellar luxation in the left stifle of 24 dogs. SF and blood samples were collected at 1.5- and 3-month intervals, respectively. Every 3 months, one dog was euthanatized to collect tissue samples from both stifles. TRAP levels in SF and serum were measured using a spectrophotometer, and TRAP-positive cells in joint tissues were identified by enzyme histochemistry. MMP-2 and TIMP-2 in SF and serum were detected by Western blotting and ELISA, respectively. TRAP in SF from the stifles and serum was significantly increased (p < 0.05) after 3 months. TIMP-2 in SF and serum was significantly decreased (p < 0.05), whereas MMP-2 in SF was significantly increased (p < 0.05) during the progression of OA. Histochemistry revealed an increased number of TRAP-positive cells in tissues from OA-affected joints. Assays measuring TRAP, MMP-2, and TIMP-2 in SF and serum, and methods that detect increased numbers of TRAP-positive cells in the joint tissues can play an important role in identifying the early phases of degenerative changes in canine joint components.
Patellar luxation (PL) is one of the most common orthopedic disorders in dogs [18]. PL can result in the development of degenerative joint disease, pain, and lameness. It has been reported that medial patellar luxation (MPL) increases the stress on the cranial cruciate ligament (CCL), predisposing the structure to degeneration and rupture. Secondary osteoarthritis (OA) is a common result of MPL [20].
OA is a slowly progressive disease with different etiologies that finally converge on the same pathogenic pathway that is hallmarked by characteristic changes in cartilage, subchondral bone, and synovial membrane. The insidious onset and "silent" progression of OA not only prevent early diagnosis of this disease, but also delay treatment that may help prevent further cartilage destruction and joint failure [16]. Hence, determinants or markers that may be used to detect and monitor molecular events early in the pathogenesis of OA are of considerable interest as to detect preclinical disease, determine prognosis, and monitor the response to drugs and therapy [10].
Although the cause of the synovial inflammation associated with OA is unknown, recent work has shown that the synovial intima contains large numbers of dendritic cells and macrophage-like cells, which express tartrate-resistant acid phosphatase (TRAP), together with increased immunoglobulin deposition [17]. TRAP-positive mononuclear cells within the synovium typically express many degradative collagenolytic enzymes including cathepsin K, cathepsin S, and matrix metalloproteinases (MMPs). The presence of TRAP-positive cells within the synovium is a characteristic feature of arthritis, and is considered to be a key factor for promoting progressive and irreversible articular cartilage and joint destruction [23]. Production of MMPs, cathepsins, and TRAP at sites of inflammation potentially contributes to articular cartilage matrix degradation [8,23]. It is well documented that osteoblasts express and secrete a large amount of TRAP. This factor has been shown to be a useful histochemical marker for identifying osteoclasts and a biochemical index for osteoclastic resorption [9,11].
Cartilage destruction is a common pathological feature in various types of arthritis, including rheumatoid arthritis (RA) and OA, and is a major cause of joint dysfunction. Two pathways are known to be involved in the destruction of cartilage. The first is an intrinsic pathway by which the chondrocytes themselves degrade cartilage extracellular matrix (ECM). The second is an extrinsic pathway through which tissues or cells other than chondrocytes, such as inflamed synovium, pannus tissue, or infiltrating inflammatory cells break down the ECM of cartilage through synovial fluid (SF). In both pathways, enzymatic digestion of the ECM results in cartilage destruction [25]. Many proteinases are expressed in joint tissues of patients with RA and OA. Among these, MMPs are believed to have a key role in the arthritic joint destruction. MMP production in fibroblasts, macrophages, and chondrocytes is mediated by synovial macrophages and lymphocytes. MMPs are the proteases that participate in ECM degradation and remodeling. The proteolytic activity of MMPs is regulated by specific inhibitors known as tissue inhibitors of metalloproteinases (TIMPs) [1,15]. The N-terminal domain of the TIMP molecule competitively binds to the active site of MMPs at a 1 : 1 ratio to form a non-covalent complex that abolishes MMP activity. TIMP-2 is the major TIMP expressed in fibroblasts, macrophages, and endothelial cells, and potently inhibits most MMPs [1,5,24].
A good biological marker of OA would be sensitive to changes in the severity of cartilage destruction. Identification of a single predictive marker is probably an unrealistic goal [4]. Current research efforts are focused on identifying combinations of several markers capable of providing information about the extent of cartilage degradation and remodeling, subchondral bone metabolism, and reactive changes in synovial and CCL tissues. However, to the best of our knowledge there is currently a lack of evidence in the literature about TRAP, MMP-2, and TIMP-2 concentrations in the SF and serum in the MPL model of OA in dogs. In order to better understanding the pathophysiology of OA and to identify possible biomarkers of early OA, the levels of TRAP and TIMP-2 in the serum and SF, as well as the level of MMP-2 in the SF, were assayed in the present study. It was hypothesized that there would be differences of the levels of these markers in the SF and serum of normal dogs and dogs with experimentally-induced OA.
The purpose of this study was to investigate whether the early phases of degenerative changes that commence after the onset of MPL can be identified by monitoring changes in the concentration of TRAP, MMP-2, and TIMP-2.
Twenty-four skeletally-mature, healthy, mixed small breed dogs of both genders, age 2 to 6 years and weighing 2.8 to 9 kg, were used for this experiment. The experimental dogs were purchased from the Preclinical Research Center (Chemon, Korea). The animals were kept in quarantine for 1 month and acclimated in cage confinement. Before participating in the experiment, physical and radiographic examinations were performed to ensure the absence of neurologic disease, patellar luxation or other orthopedic diseases, or to determine the animals had previously undergone surgical procedures involving either hind limb. The dogs were housed in individual cages in the departmental animal shed at the Department of Surgery, College of Veterinary Medicine, Chonbuk National University, Korea. They were fed a standard commercial diet (Precept, USA) and had access to water ad libitum.
After the animals were premedicated with atropine sulfate (0.05 mg/kg, SC; Dai Han Pharm, Korea), anesthesia was induced with thiopentone sodium (25 mg/kg, IV; Dai Han Pharmaceutical, Korea) and maintained with enflurane and oxygen delivered through a cuffed endotracheal tube.
In all of the experimental animals (n = 24), left stifle arthrotomy was performed using a standard parapatellar approach. An incision was made through the fascia lata just lateral to the patellar ligament. The joint was thoroughly examined to evaluate the condition of the cruciate ligaments and menisci. MPL was induced by placing purse string sutures around the parapatellar fibrocartilage and anchoring the patella to the fabellar ligament using monofilament sterile nylon sutures. Medial retinacular reinforcement (imbrication) and lateral release were also performed to interfere with the neutral tracking of the patella. Dogs in the sham group (n = 12) underwent sham operation on their right stifles, whereas animals in the non-surgical control group (n = 12) remained intact. For the sham operation, a scalpel blade was inserted into the right stifle through a lateral parapatellar stab incision to inflict minor trauma to the synovium while the other joint components remained intact.
Post-surgically, the animals were kept in close observation in a postoperative room and were administered cephalexin (25 mg/kg, IV; Union Korea Pharmaceutical, Korea) every 8 h, for 5 days and dexamethasone (0.2 mg/kg, IV; Daewon Pharmaceutical, Korea) every 6 h for 3 days. The external stitches were removed after the surgical wounds healed, and the animals were moved to the experimental animal shed after 10 days.
SF specimens were collected preoperatively and postoperatively at 1.5 month intervals from both stifles (index and contralateral). As much SF was collected as possible (0.2~0.45 mL) by arthrocentesis from the stifle joint and special care was taken to avoid contamination with blood. After noting the volume and appearance of the SF, the specimens were centrifuged at 12,000 × g for 10 min at 4℃ to remove the cells, and the supernatant was stored at -80℃ until biomarker assays were performed.
Blood samples were collected prior to and 3, 6, 9, and 12 months after experimental induction of OA, and subjected to TRAP and TIMP-2 assays. Three mL of blood samples from each animal were collected using a 5 mL disposable syringe (Hwajin Medical, Korea) and transferred to sterile screw-capped tubes without anticoagulant (Soyagreentec, Korea), left undisturbed at room temperature for 1 h to permit coagulation, and centrifuged at 1,500 × g for 15 min. The serum was collected and transferred to Eppendorf tubes and stored at -80℃ until it was used for the assays.
TRAP activity in the SF and serum specimens was measured using a biochemical assay in 96-well plates with para-nitrophenylphosphate (pNPP) (Sigma-Aldrich, USA) as a substrate. All reagents for this assay were purchased from a commercial supplier. To reduce sample viscosity and improving pipetting accuracy, the SF specimens were digested with 50 IU/mL of streptomyces hyaluronidase (Sigma-Aldrich, USA) at at 37℃ for 30 min prior to the assay. The serum specimens were also incubated at 37℃ without streptomyces hyaluronidase for 30 min to inactivate the acid phosphatase activity of the erythrocytes. All of the SF and serum samples were diluted 1:4 with 0.9% NaCl before the assay. The 0.9% NaCl solution was used as the negative control. SF and serum samples (100 µL) were added to 100 µL of the reaction mixture so that the final incubation medium contained 2.5 mM pNPP, (ditris salt), 0.1 M sodium acetate buffer, pH 5.8; 0.2 M KCl, 0.1% Triton X-100, 10 mM sodium tartrate, 1 mM ascorbic acid, and 100 µM FeCl3. The concentration of TRAP was determined using 200 µL of the final incubation medium per well in the 96-well plate (Immulon 2 HB Flat Bottom Microtiter Plate; Dynex Technologies, USA). After incubating the 96-well plate for 1 h at 37℃, the liberated p-nitrophenol was converted to p-nitrophenolate by adding 50 µL of 0.9 M NaOH, and the absorbance was measured at 405 nm using a spectrophotometer (Cess UV 90c; Bioteck, USA). One unit (U) of TRAP activity corresponded to 1 mol of p-nitrophenol liberated per 1 min at 37℃. If the TRAP activity exceeded the range of the standard curve, the samples were further diluted and assayed.
SF and serum samples were assayed for TIM-2 activity using a commercially available TIMP-2 Biotrak ELISA system (Amersham, UK). The samples were assayed according to the protocol provided by the kit manufacturer. Briefly, 100 µL of the prepared standard and 100 µL of each sample were incubated at 25℃ for 1 h with 100 µL of the anti-TIMP-2 peroxidase conjugate. The wells were then aspirated, washed, and incubated with 100 µL of tetramethylbezidine hydrogen peroxide at 25℃ for 30 min. This reaction was stopped by the addition of 1.0 M sulphuric acid and the optical density was measured at 450 nm using a spectrophotometer (Cess UV 90c; Bioteck, USA). The concentrations of TIMP-2 were determined according to a standard curve and reported in ng/mL.
SF samples (40 µL) were incubated in Laemmli sample buffer (LSB) with β-mercaptoethanol (40 µL), heated to 90℃ for 5 min, and separated by SDS polyacrylamide gel electrophoresis on 4~20% Tris-glycine gradient gels (Bio-Rad Laboratories, USA). A sample of human MMP-2 protein (3 µL; Sigma-Aldrich, USA) was mixed in LSB (3 µL), heated to 90℃ for 5 min, and run on each gel as a control. The separated proteins were then electrophoretically transferred to a polyvinylidene difluoride (PVDF) membrane (Sigma-Aldrich, USA) in a tank system with plate electrodes. The membranes were blocked for 1 h at 25℃ in 5% non-fat dried milk in TBS-Tween, and then incubated in room temperature for 1 h with mouse monoclonal anti-MMP-2 antibody (1:1,000 dilution in TBS-Tween; Sigma-Aldrich, USA). After incubating, the membranes were washed four times with 0.1% TBS-Tween, and then incubated for 1 h at room temperature with a horseradish peroxide-conjugated secondary antibody (Amersham, UK) diluted 1 : 10,000 in TBS-Tween with 0.1% Tween. After four washes with TBS-Tween, the immunoreactive bands were visualized with Pico chemiluminescent substrate (Pierce Biotechnology, USA) and the blots were exposed to radiographic film (Pierce Biotechnology, USA).
Histochemical staining to visualize TRAP expression was performed on 15 µm frozen sections of the CCL and synovium specimens as described by Muir et al. [17]. All reagents for histochemical staining were obtained from Sigma-Aldrich (USA). A solution of naphthol AS-BI phosphate was prepared by dissolving 25 mg of naphthol AS-BI phosphate in 2.5 mL of n,n-dimethylformamide to which 45 mL of 0.05 M Tris-maleate buffer (pH 5) was added. A solution of hexazotized pararosanaline was prepared by dissolving 0.25 g of pararosaniline hydrochloride in 5 mL of distilled water and then adding 1.25 mL of hydrochloric acid. This solution was mixed with an equal volume of 4% sodium nitrite immediately before use. The final reaction mixture for histochemical staining was prepared by adding 4 mL of the hexazotized pararosanaline solution to the naphthol AS-BI phosphate solution along with 50 mM sodium-potassium tartrate. The final reaction mixture was filtered using syringe filters (Nalge, USA) before use. The 15 µm thick sections were incubated in the reaction mixture at 37℃ for 1~2 h, rinsed in distilled water, counterstained with Mayer's hematoxylin, and mounted. For each batch of slides, a negative control was prepared by omitting the naphthol AS-BI phosphate. The CCL and synovium specimens were examined by light microscopy for presence of the cells that contained TRAP.
The Western blots were scanned using a digital imaging system (Fusion FX7; Vilber Lourmat, Germany) and the relative intensity of the bands was determined by densitometry (Bio-Rad Laboratories, USA). The data obtained in the present study were analyzed using an ANOVA and Student's t-test. A p value < 0.05 was considered to be statistically significant. The data are presented as the mean ± SD.
The level of TRAP in the SF in OA-induced stifles increased significantly (p < 0.05) 3 months postoperatively in comparison to that of the pre-surgical control, post-surgical contralateral control and sham-operated stifles. This elevated level was sustained throughout the experimental period (Fig. 1). The serum level of TRAP was found to be increased 3 months postoperatively and was significantly increased (p < 0.05) 6 months after the induction of OA (Fig. 2).
The level of TIMP-2 in the SF collected from OA-induced stifles was significantly decreased (p < 0.05) 3 months postoperatively compared to the pre-surgical control, post-surgical contralateral control, and sham-operated stifles (Fig. 3). The serum level of TIMP-2 was decreases 3 months postoperatively and significantly decreased (p < 0.05) 6 months after the induction of OA (Fig. 4).
The level of MMP-2 in the SF was found to increase significantly (p < 0.05) on 3 months after induction of OA and the levels observed on the subsequent experimental period were significantly (p > 0.05) above the control value (Fig. 5).
Histochemical staining specific for TRAP revealed the presence of TRAP-positive cells in the synovium, CCL, and cartilage 3 months postoperatively in the OA-induced stifles but not in the contralateral control or sham-operated stifles (Figs. 6, 7, 8). The synovium, CCL, and cartilage collected from the index stifles after 6 and 12 months of OA induction showed markedly increased numbers of TRAP-positive cells. Moderate synovial inflammation is also seen as mononuclear cell infiltration into the synovium. However, the contralateral control and shamoperated stifles also showed a mild increase in the number of TRAP-positive cells in the synovium and CCL tissues during the later stage of OA.
OA is a disease with many complex etiologies and affects all adjacent tissues in diarthrodial joints [21]. Morphological, biochemical, structural, and biomechanical changes of the ECM and cells are observed in cases of OA which lead to the degeneration of articular cartilage with softening, fibrillation, ulceration, and loss of cartilage tissues. Since the functionality of diarthrodial joints cannot be sustained without articular cartilage, the precise and early diagnosis of OA is fundamental to preventing or reducing long-term disability. Since the degenerative processes that lead to OA begin long before clinical manifestations of the disease, early diagnosis of OA is challenging [7,16]. Recently, investigations have focused on finding SF or serum biomarkers that may be useful for diagnosing, monitoring treatment efficacy, or determining prognosis for patients with OA [7].
TRAP contains a binuclear iron center with two iron molecules, one that is redox-active and the other that stabilizes the ferric form. In addition to its phosphatase activity, TRAP is also capable of producing reactive oxygen species (ROS) through Fenton's reaction in which the redox-active ferrous iron reacts with hydrogen peroxide to generate highly destructive hydroxyl radicals. This activity has been detected by three different laboratories using three different methods [8,22]. ROS generated by TRAP are capable of destroying type I collagen, the main protein in bone matrix. Macrophages overexpressing TRAP increase the amounts of intracellular ROS, suggesting that the enzyme is capable of producing ROS in vivo [8]. ROS generation is optimal at a neutral pH, distinguishing this process from phosphatase activity that is optimal at an acidic pH [12]. This suggests that these two activities may function in different intracellular compartments, and that the pH of the environment may determine which activity is involved. Mutant TRAP enzymes that are completely inactive as phosphatase are capable of producing ROS, indicating that these two activities are functionally independent. In the present study, the significant increase in the level of TRAP in SF and serum as well as increased numbers of TRAP-positive cells in the joint tissues during the early stage of OA indicated that TRAP might have a potential role in the pathogenesis of this disease [14].
TIMPs are known as endogenous protease inhibitors that bind to active MMPs at a 1 : 1 molar ratio [1,3,15]. Although the pathogenesis of OA is not fully understood, MMPs derived from chondrocytes, synovium, and polymorphonuclear leukocytes have been proposed to play a major role in cartilage degradation seen in cases of OA. The balance between MMPs and TIMPs is tightly controlled in healthy joints. However, in OA, the amount of MMPs exceeds the locally-available levels of TIMPs, resulting in excessive ECM degradation [6,19]. The N-terminal domain of the TIMP molecule competitively binds to the active site on the MMP enzyme at a 1 : 1 ratio to form a non-covalent complex that abolishes MMP activity. TIMP-2 is the major TIMP expressed by fibroblasts, macrophages, and endothelial cells, and potently inhibits most MMPs [1,5,24]. Significant increases in MMP-2 levels with a simultaneous decrease in TIMP-2 levels in the SF observed in the present study suggested a disruption in the balance of these two enzymes, thereby indicated a high rate of turnover in articular cartilage. These findings are in agreement with those of previous reports [14,21,25]. Decreased serum levels of TIMP-2 with the progression of the disease further correlated with the levels of this enzyme in the SF. This finding contrasts with the report by Salinardi et al. [21], but is in complete agreement with the report by Klimiuk et al. [13].
The ELISA 'sandwich' system used for this study to measure TIMP-2 concentrations recognized both free TIMP-2 and enzyme that was complexed with the active form of MMPs. The immunoreactivities of free and complexed TIMP-2 were 100% and 330%, respectively. This ELISA did not recognize TIMP-1 or -3; therefore, cross-reactivity with other TIMPs was very low. The ELISA used to measure TIMP-2 is capable of quantifying TIMP-2 with a sensitivity defined as two standard deviations above the mean optical density. The TIMP-2 peptide epitopes used to produce antibodies for the ELISA are conserved across different species. Therefore, the assay should recognize TIMP-2 from all species including human, mouse, rat, dog, guinea pig, and cow [2,5].
Changes in the SF and serum levels of TIMP-2 and SF levels of MMP-2 found during different periods after OA induction indicates that these enzymes may be involved in the pathogenesis of OA. This finding may provide information about the progression and degree of degenerative changes during the disease process. However, this idea cannot be concluded strictly as the pathogenesis and mechanism, how the degenerative changes occur is not yet clearly understood. The findings regarding the changes in the levels of TIMP-2 and MMP-2 with the progression of OA is much informative to support them as biomarkers for the detection of the early stages of the disease. Nevertheless, the results of this study broaden the knowledge about TRAP, MMP-2 and TIMP-2, and suggest the necessity for further studies to explore the role of these enzymes as therapeutic targets for treating canine OA. The changes in the levels of TRAP, MMP-2 and TIMP-2 in the SF and serum with OA progression as well as the presence of TRAP-positive cells in the joint tissues imply that these enzymes may also serve as biomarkers to identify the early stages of OA in dogs.
Figures and Tables
 | Fig. 1Tartrate resistant acid phosphatase (TRAP) levels in the synovial fluid (SF) of the index and contralateral (control and sham) stifles after induction of osteoarthritis (OA) during different experimental periods. |
 | Fig. 2TRAP levels in the serum during different experimental periods after inducing OA secondary to medial patellar luxation (MPL). |
 | Fig. 3Tissue inhibitor of matrix metalloproteinase-2 (TIMP-2) levels in the SF of the index and contralateral (control and sham) stifles during different experimental periods after OA induction. |
 | Fig. 4TIMP-2 levels in the serum at different experimental periods after inducing OA secondary to MPL. |
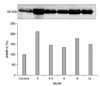 | Fig. 5Western blot showing matrix metalloproteinase-2 (MMP-2) levels in the SF during different experimental periods after the induction of OA secondary to MPL. |
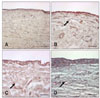 | Fig. 6Microphotographs of the stained frozen synovium section. The red mononuclear cells (arrow) are positive for TRAP. (A) Control. (B) OA 3 month. (C) OA 6 month. (D) OA 12 month. Scale bars = 100 µm. |
Acknowledgments
This work was funded by a Postdoctoral Fellowship provided to the first author from Chonbuk National University (Korea).
References
1. Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen D, DeCarlo A, Engler JA. Matrix metalloproteinases: A review. Crit Rev Oral Biol Med. 1993. 4:197–250.

2. Catterall JB, Cawston TE. Assays of matrix metalloproteinases (MMPs) and MMP inhibitors: bioassays and immunoassays applicable to cell culture medium, serum, and synovial fluid. Methods Mol Biol. 2003. 225:353–364.

3. Cawston TE. Metalloproteinase inhibitors and the prevention of connective tissue breakdown. Pharmacol Ther. 1996. 70:163–182.

4. Chevalier X. Is a biological marker for osteoarthritis within reach? Rev Rhum Engl Ed. 1997. 64:562–577.
5. Clegg PD, Coughlan AR, Carter SD. Equine TIMP-1 and TIMP-2: Identification, activity and cellular sources. Equine Vet J. 1998. 30:416–423.

6. Dean DD, Martel-Pelletier J, Pelletier JP, Howell DS, Woessner JF Jr. Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J Clin Invest. 1989. 84:678–685.

7. Fox DB, Cook JL. Synovial fluid markers of osteoarthritis in dogs. J Am Vet Med Assoc. 2001. 219:756–761.

8. Halleen JM, Räisänen S, Salo JJ, Reddy SV, Roodman GD, Hentunen TA, Lehenkari PP, Kaija H, Vihko P, Väänänen HK. Intracellular fragmentation of bone resorption products by reactive oxygen species generated by osteoclastic tartrate-resistant acid phosphatase. J Biol Chem. 1999. 274:22907–22910.

9. Hayman AR, Bune AJ, Bradley JR, Rashbass J, Cox TM. Osteoclastic tartrate-resistant acid phosphatase (Acp 5): Its localization to dendritic cells and diverse murine tissues. J Histochem Cytochem. 2000. 48:219–228.

10. Hegemann N, Kohn B, Brunnberg L, Schmidt MF. Biomarkers of joint tissue metabolism in canine osteoarthritic and arthritic joint disorders. Osteoarthritis Cartilage. 2002. 10:714–721.

11. Janckila AJ, Simons RM, Yam LT. Alternative immunoassay for tartrate-resistant acid phosphatase isoform 5b using the fluorogenic substrate naphthol ASBI-phosphate and heparin. Clin Chim Acta. 2004. 347:157–167.

12. Kaija H, Alatalo SL, Halleen JM, Lindquist Y, Schneider G, Vaananen HK, Vihko P. Phosphatase and oxygen radical-generating activities of mammalian purple acid phosphatase are functionally independent. Biochem Biophys Res Commun. 2002. 292:128–132.

13. Klimiuk PA, Sierakowski S, Latosiewicz S, Latosiewicz R, Cylwik B, Skowronski J, Chweicko J. Serum matrix metalloproteinases and tissue inhibitors of metalloproteinases in different histological variants of rheumatoid synovitis. Rheumatology (Oxford). 2002. 41:78–87.

14. Lee HB, Alam MR, Seol JW, Kim NS. Tartrate-resistant acid phosphatase, matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 in early stages of canine osteoarthritis. Vet Med (Praha). 2008. 53:214–220.

15. Matrisian LM. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990. 6:121–125.

16. Matyas JR, Atley L, Ionescu M, Eyre DR, Poole AR. Analysis of cartilage biomarkers in the early phases of canine experimental osteoarthritis. Arthritis Rheum. 2004. 50:543–552.

17. Muir P, Schamberger GM, Manley PA, Hao Z. Localization of cathepsin K and tartrate-resistant acid phosphatase in synovium and cranial cruciate ligament in dogs with cruciate disease. Vet Surg. 2005. 34:239–246.

18. Ness MG, Abercromby RH, May C, Turner BM, Carmichael S. A survey of orthopaedic conditions in small animal veterinary practice in Britain. Vet Comp Orthop Traumatol. 1996. 9:43–52.

19. Pelletier JP, Mineau F, Faure MP, Martel-Pelletier J. Imbalance between the mechanisms of activation and inhibition of metalloproteases in the early lesions of experimental osteoarthritis. Arthritis Rheum. 1990. 33:1466–1476.

20. Roy RG, Wallace LJ, Johnston GR, Wickstrom SL. A retrospective evaluation of stifle osteoarthritis in dogs with bilateral medial patellar luxation and unilateral surgical repair. Vet Surg. 1992. 21:475–479.

21. Salinardi BJ, Roush JK, Schermerhorn T, Mitchell KE. Matrix metalloproteinase and tissue inhibitor of metalloproteinase in serum and synovial fluid of osteoarthritic dogs. Vet Comp Orthop Traumatol. 2006. 19:49–55.

22. Sibille JC, Doi K, Aisen P. Hydroxyl radical formation and iron-binding proteins. Stimulation by the purple acid phosphatases. J Biol Chem. 1987. 262:59–62.

23. Tsuboi H, Matsui Y, Hayashida K, Yamane S, Maeda-Tanimura M, Nampei A, Hashimoto J, Suzuki R, Yoshikawa H, Ochi T. Tartrate resistant acid phosphatase (TRAP) positive cells in rheumatoid synovium may induce the destruction of articular cartilage. Ann Rheum Dis. 2003. 62:196–203.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download