Abstract
The objective of this study was to characterize acrosomal ultrastructure following discontinuous Percoll gradient centrifugation of cryopreserved bovine sperm. Semen was collected from six bulls of different breeds and three ejaculates per bull were evaluated. Frozen semen samples were thawed and the acrosomal region of sperm cells was evaluated by transmission electron microscopy (TEM) before (n = 18) and after (n = 18) Percoll centrifugation. The evaluation of 20 sperm heads from each of the 36 samples analyzed ensured that a large number of cells were investigated. The data were subjected to analysis of variance at a level of significance of 5%. Percoll centrifugation reduced the percentage of sperm exhibiting normal acrosomes (from 61.77 to 30.24%), reduced the percentage of sperm presenting atypical acrosome reactions (from 28.38 to 4.84%) and increased the percentage of sperm exhibiting damage in the acrosome (from 6.14 to 64.26%). The percentage of sperm with typical acrosome reactions was not significantly different before (3.70%) and after (0.67%) centrifugation. TEM distinguished four different types of acrosomal status and enabled ultrastructural characterization of acrosomal injuries. The percentage of sperm exhibiting normal acrosomes decreased and damage in the acrosome was the most frequent acrosomal injury with the Percoll gradient centrifugation protocol utilized.
Selecting good quality sperm from frozen-thawed semen is an important factor for successful in vitro fertilization (IVF). Density gradients, especially Percoll, are recognized as one of the best and most commonly used methods for sperm selection [4,12,13,19].
Percoll consists of colloidal silica particles coated with polyvinylpyrrolidone (PVP), which selects sperm according to their maturation stage and integrity [17] and acts as an artificial barrier to low density [10,17] and immotile cells [20]. Consequently, colloidal Percoll solutions allow the separation of impurities (light particles, immature sperm, debris, dead cells) from motile sperm in a single centrifugation step. The impurities and immotile cells are retained in the upper portions of the gradient while the pellet with selected sperm is deposited in the area of greater density [10].
As Percoll separation methods are in widespread use in bovine IVF [4,12], numerous papers concerning the impact of this method on sperm have been published [4,12,14,16]. Several authors are in agreement that the percentage of sperm with intact plasma membrane is improved after Percoll treatment of frozen-thawed bull semen [4,12,14,16]. However, the results are contradictory regarding acrosomal membrane integrity [2,4,12,14,16,22].
The mammalian acrosome is a Golgi-derived sperm compartment that contains hydrolytic enzymes and lies as a cap over the anterior portion of the sperm nucleus [5]. The acrosome reaction is an essential event to sperm penetration into the zona pellucida and fertilization. During the acrosome reaction, acrosomal enzymes are released and much of the anterior head membranes are lost as hybrid vesicles of the plasma membrane and the outer acrosomal membrane [5]. Thus, the sperm cell must maintain an intact acrosome up to the time it binds to the zona pellucida [8,18].
Arcidiacono et al. [2] demonstrated that centrifugation in a Percoll gradient can damage the acrosomal membrane. Also, Oliveira et al. [16] demonstrated that the percentage of sperm cells presenting intact acrosomal membranes was severely reduced after Percoll centrifugation. However, the question remains whether Percoll density gradient centrifugation induces acrosome reactions or whether injuries in the acrosome are caused by the process. Therefore, the objective of the present study was to characterize the acrosomal ultrastructure after discontinuous Percoll density gradient centrifugation on cryopreserved bovine sperm using transmission electron microscopy (TEM).
All frozen semen doses diluted in Tris-egg yolk extender were purchased from the same company, which specializes in freezing and commercialization of semen. Semen from six bulls of different breeds (Angus, Holstein, Jersey, Gir, Guzerá and Nelore) was used and three different ejaculates were evaluated for each animal. The frozen-thawed semen samples were evaluated by TEM before (control group) and after discontinuous Percoll density gradient centrifugation (centrifuged group).
A 90% Percoll solution (pH 7.4; osmolarity 280~290 mOsm/kg H2O) was prepared by diluting nine parts Percoll (Sigma-Aldrich, USA) in one part (1 : 9, v/v) Dulbecco's modified Eagle's medium 10× concentrated (DMEM 10×; Gibco, USA), complemented with 0.01 g/L gentamicin (Gibco, USA) and 6 mM HEPES (Sigma-Aldrich, USA). The DMEM 10× was prepared by diluting the amount of powder needed to prepare 1 L in 100 mL of double-distilled ultrapure water (Milli Q System; Millipore, USA). The solution was then filtered through a 0.22-µm membrane and stored at 4℃ for 3 days. A 1× concentrated DMEM solution was also prepared according to the manufacturer's instructions and filtered through a 0.22-µm membrane and stored at 4℃ for 3 days. The Percoll solutions with different densities were prepared by diluting different proportions of 90% Percoll solution in DMEM 1× medium containing 0.3% bovine serum albumin (BSA) fraction V (Calbiochem, Germany). Three isotonic (280~290 mOsm/kg) Percoll solutions with densities of 1.110 g/mL (80% Percoll solution), 1.115 g/mL (85% Percoll solution) and 1.123 g/mL (90% Percoll solution) were obtained. Percoll suspensions were prepared one day before the experiment and stored at 4℃. The discontinuous gradient was obtained by layering 2 mL of decreasing density solutions from bottom to top in 15 mL polystyrene tubes (Corning, USA). One discontinuous Percoll gradient tube was prepared for each semen ejaculate.
For each ejaculate, four semen straws (0.25 mL) were thawed at 37℃ for 20 sec and then homogenized. Part of this sample (0.5 mL) was placed in the tube containing the Percoll density gradient. Aliquots of 200 µL from the remaining sample were fixed for subsequent assessment as the control group. The tubes containing the density gradient and semen were centrifuged at 500× g in a swing-out rotor (refrigerated Sorvall RC-3C Plus centrifuge; Sorvall Products, USA) at 22℃ for 20 min. After centrifugation, the supernatant was removed. The pellet containing the sperm cells (200 µL per tube) was recovered and fixed for subsequent assessment of the centrifuged group. The pellets were resuspended directly in the fixative solution.
Before and after sperm selection by Percoll gradient centrifugation, samples (200 µL) were fixed with 4% glutaraldehyde in Dulbecco's modified phosphate buffered saline (Sigma-Aldrich, USA) solution (300 mOsm/kg) for 1 h. Samples were postfixed in 1% OsO4 overnight, dehydrated, embedded in Epon resin (Epon 812; Fluka Chemie, Switzerland) and sectioned for TEM. Ultrathin sections were observed at 80 kV using a Zeiss 109 electron microscope (Carl Zeiss, Germany). The acrosomal status of 20 sperm cells per sample was evaluated.
Sperm head evaluation by TEM was based on Saacke and Marshall [21], and Cross and Meizel's [5] observations. A total of 20 sperm heads per ejaculate were examined (60 per bull), and 18 control samples (n = 360) and 18 centrifuged samples (n = 360) were analyzed. The evaluation of 20 sperm cells from each of the 36 test subjects (in all, n = 720) ensured that a large number of cells was investigated. According to the acrosomal characteristics observed, it was possible to classify the sperm into four different categories: 1) sperm with normal acrosomes (sperm head exhibiting intact acrosomal membrane completely surrounding the acrosomal ground substance; Fig. 1A), 2) sperm with a "physiological" acrosome reaction (sperm head exhibiting swelling of acrosomal ground substance with vesicles of fused plasma and outer acrosomal membranes; Fig. 1B), 3) sperm exhibiting an atypical acrosome reaction (sperm head presenting swelling of acrosomal ground substance dispersed under the swollen outer acrosomal membrane; Fig. 1C), and 4) sperm presenting damage in the acrosome (sperm head exhibiting rupture of the acrosomal membrane with swelling of acrosomal ground substance in restricted points; Fig. 1D). Sperm with an absent acrosome or when status could not be easily discerned were not included in the count.
The results were analyzed using the SAS program (SAS Institute, USA). The variables were subjected to ANOVA at a level of significance of 5% for the determination of qualitative differences in acrosomal status between the semen samples of the control (frozen-thawed sperm) and centrifuged groups (frozen-thawed sperm subjected to Percoll density gradient centrifugation).
The percentage of sperm presenting normal acrosomes was reduced (from 61.77 ± 2.49% to 30.24 ± 7.78%; p < 0.01). The percentage of sperm presenting a physiological acrosome reaction was not significantly different before (3.70 ± 1.22%) and after (0.67 ± 0.67%) centrifugation. In addition, Percoll gradient centrifugation reduced (p < 0.01) the percentage of sperm exhibiting atypical acrosome reactions (from 28.38 ± 3.04% to 4.84 ± 3.09%) and increased (p < 0.01) the percentage of sperm presenting damage in the acrosome (from 6.14 ± 1.93 % to 64.26 ± 9.97%). The results of acrosomal characteristics evaluated by TEM in control and centrifuged groups are presented in Table 1.
There are a number of sperm selection techniques for use with bovine sperm. These techniques are used for removing seminal plasma, dead cells, abnormal sperm, cryoprotective agents, and other factors [15]. The techniques include Percoll density gradient centrifugation [4,7,12-15,19,22], swim-up migration [4,7,15], washing by centrifugation [7,15], glass wool filtration [15], and many other.
Acrosome integrity, as well as enzyme maintenance, is crucial to successful fertilization [8,18]. When the acrosomal membrane is injured, the penetration of specific molecules is facilitated, allowing for detection of damaged acrosomes [3,25]. Oliveira et al. [16] used a fluorescein isothiocyanate-conjugated Pisum sativum agglutinin (FITC-PSA) fluorescent probe [3,6] to assess acrosomal integrity. The authors observed that the percentage of frozen-thawed sperm cells with intact acrosomal membranes was markedly reduced after centrifugation in a discontinuous gradient prepared with 2 mL of 80%, 2 mL of 85%, and 2 mL of 90% Percoll solution. This raised the question of whether Percoll centrifugation induces the acrosome reaction or whether other injuries are caused in the acrosome.
TEM is typically the standard against which a new assay is measured [5]. In the present study, TEM revealed four different ultrastructural acrosome statuses following Percoll gradient centrifugation. According to the acrosomal characteristics observed, the percentage of frozen-thawed sperm exhibiting intact acrosomes was reduced after Percoll centrifugation from 61.8% in the control group to 30.2% in the centrifuged group. This finding is in agreement with a study by Oliveira et al. [16] which reported that the percentage of sperm cells with intact acrosomal membranes was reduced from 85.0% (bovine frozen-thawed semen) to 45.1% following Percoll centrifugation.
In contrast, Machado et al. [12], Mehmood et al. [14], and Somfai et al. [22] reported that the proportion of frozen-thawed bull sperm with intact acrosomes increased after centrifugation using a gradient of 2 mL of 45% over 2 mL of 90% Percoll solution. Cesari et al. [4] demonstrated by TEM that the percentage of sperm with partially lost acrosomes after centrifugation in a Percoll gradient (prepared with 0.5 mL of 30%, 0.5 mL of 60%, and 0.5 mL of 90% Percoll solution) was not different from the percentage of sperm with partially lost acrosomes in post-thawed semen.
In the present study, damage in the acrosome appears to be the most frequent injury after Percoll centrifugation. The occurrence of damage in the acrosome increased from 6.1% in the control group to 64.3% in the centrifuged group. Percoll migration imposes mechanical contact with the cells [12] and the centrifugation is a potential sperm damaging step [1]. Therefore, the centrifugation force used on a Percoll gradient may affect sperm quality [26]. Differences in centrifugal force between the present study and the studies previously mentioned were also observed. In the study by Somfai et al. [22], the gradient was centrifuged for 10 min at 300× g. In the Cesari et al. [4] study, the gradient was centrifuged for 10 min at 700× g. Mehmood et al. [14] centrifuged the gradient for 15 min at 700× g while Machado et al. [12] centrifuged a 4 mL gradient for 20 min at 700× g, as well as a 800 µL gradient for 20 min at 700× g or for 5 min at 5,000× g, and observed no effect of the centrifugation protocol on sperm quality. In the present study, the gradient was centrifuged for 20 min at 500× g. Hence, with the exception of Somfai et al. [22], the studies mentioned used similar centrifugal force as that used in the present work. Thus, the differences in centrifugal forces do not appear to be responsible for the acrosomal damage observed in this experiment.
On the other hand, the differences in Percoll layers between the present study and the other studies could be an explanation for the divergences in results regarding the acrosomal status after centrifugation. The increased height and density of the Percoll gradient used in the present work may have offered an extra challenge to sperm cells resulting in prolonged sperm migration and prolonged contact with Percoll. This finding reinforces the idea that PVP present in silica-based density gradients can be damaging to sperm membranes [13,24]. In human sperm, PVP has been shown to cause plasma membrane damage, acrosome damage, and mid-piece damage without centrifugation [24]. In addition, the high sperm concentration within the pellet containing silica-based medium may have contributed to the occurrence of ultrastructural damage to sperm cells [23]. In this respect, Arcidiacono et al. [2] demonstrated by TEM that Percoll seems to induce swelling of the cell membrane in the acrosomal region as well as provoke the occurrence of vacuoles in the acrosome.
Sterzik et al. [23] demonstrated by scanning electron microscopy that the percentage of sperm cells presenting intact acrosomes increased from 15% in control semen (human fresh semen) to 33% after Percoll centrifugation (with a gradient composed of 0.5 mL of 95% and 0.5 mL of 45% Percoll solutions). The percentage of slightly damaged acrosomes did not change before (15%) or after (15%) centrifugation and the percentage of severely damaged acrosome slightly increased from 25% (control) to 30% after Percoll centrifugation [23]. However, it is important to note that Sterzik et al. [23] used only fresh semen, while in the present study only cryopreserved semen was subjected to Percoll centrifugation. The freezing and thawing processes appear to reduce the protection of sperm cell membranes and to facilitate sperm capacitation [9,11], a fact that contributes to acrosomal injuries. Moreover, the prolonged contact with Percoll could have induced some alterations such as membrane destabilization and faster capacitation [4]. These factors may have further facilitated the incidence of acrosomal injury after Percoll centrifugation.
The acrosome reaction, a modified form of exocytosis, profoundly changes the sperm both structurally and functionally. The sperm must first undergo the process of capacitation before they can undergo the acrosome reaction [5]. Cesari et al. [4] stated that the colloidal particles adhere to the sperm membranes removing some decapacitating proteins from the sperm surface during their passage through a Percoll gradient. Also, the authors suggest that this factor can be one explanation why a high percentage of Percoll selected sperm are capable of undergoing acrosomal reactions. However, in the present study, the percentage of sperm cells presenting physiological acrosome reactions was not significantly different in the control (3.7%) and centrifuged group (0.67%). The criterion used in this study to classify physiological acrosome reactions requires that the hybrid vesicles be obviously present. These hybrid vesicles can only be seen in the initial stage of the acrosome reaction [5]. Sperm with absent acrosome or sperm of indiscernible classification were removed from the count in this experiment. Hence, it is possible that some sperm cells exhibiting physiological acrosome reactions were not considered. Nevertheless, according to the observations of the present experiment, it is more likely that acrosomal damages rather than physiological acrosome reactions occurred with the sperm selection procedure used.
The percentage of sperm presenting atypical acrosome reactions was significantly reduced following the Percoll separation method used in the present study (from 28.4% in the control to 4.8% in the centrifuged group). This is interesting data since this atypical acrosome reaction observed by TEM is probably a false or degenerative acrosome reaction, which follows cell death [5]. In this case, most sperm classified as having atypical acrosome reactions would be dead or dying cells with acrosomes in the process of degeneration. Therefore, the reduction of this type of sperm cell after Percoll centrifugation confirms the selective property of Percoll, demonstrating that most dead sperm present in frozen-thawed semen are retained in the upper layers of the Percoll gradient.
As stated previously, several sperm separation methods have been developed [15] but Percoll centrifugation has remained the most commonly used, mainly due to the improvements in sperm characteristics that it provides [4,12-14,19,22]. Also, Percoll gradient is the preferred method to prepare frozen-thawed bovine sperm for IVF because it yields higher motility and a higher number of insemination doses [4]. In addition, no differences in fertilization rates have been reported among Percoll gradient and other typical sperm separation methods [7].
In the present study, the increase in the percentage of sperm cells with damage in the acrosome indicates a negative effect of Percoll centrifugation on frozen-thawed sperm quality. However, it is important to note that the volume and density of Percoll gradient used in this study was higher than those used in previous studies. Since it is recognized that the PVP present in Percoll medium can affect the structure of treated spermatozoa [24], it may be considered that acrosomal damage is in fact lower when Percoll gradients with lower volumes are used. On the other hand, it is possible that the high capacity of Percoll for selecting good quality sperm for embryo production [4] associated with the sufficiently large sperm dose used for IVF could compensate for the higher number of sperm with damage in the acrosome and consequently, the fertility rate would not be affected. However, given that IVF was not performed as part of the present work, this hypothesis can not be confirmed. In this sense, it is worth mentioning that Cesari et al. [4] obtained a higher percentage of sperm with partially lost acrosomes after Percoll centrifugation compared to the swim-up treatment, although no difference in embryo production was observed among the groups [4].
Nevertheless, the present experiment provides evidences that Percoll centrifugation can be damaging to the acrosome, especially when density gradients consisting of large volumes are used. Hence, in this case, the risk of such acrosomal injuries in assisted reproduction must be considered. Also, it is noteworthy that strategies to prevent the occurrence of acrosomal damages during freezing and thawing processes, as well as during Percoll centrifugation, such as adding decapacitating agents to the semen before centrifugation, may protect the sperm from the undesired effects of some selection procedures. However, further studies are needed to verify whether the differences in results regarding acrosomal damage following Percoll centrifugation are a consequence of different compositions and/or volumes of Percoll layers, or of differences in the centrifugation protocol used.
In conclusion, using TEM we were able to distinguish four different types of acrosomal status and effectively characterize the ultrastructure of acrosomal injuries. The procedure and/or the specific Percoll gradient were responsible for a reduction in the percentage of sperm exhibiting normal acrosomes. Furthermore, damage in the acrosome appears to be the most frequent acrosomal injury found in conjunction with the Percoll gradient centrifugation protocol utilized in this study.
Figures and Tables
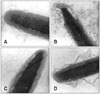 | Fig. 1Electron micrographs of a sagittal section of a bovine sperm head showing a spermatozoon. (A) Normal acrosome, sperm head presenting an intact acrosomal membrane completely surrounding the acrosomal ground substance. (B) "Physiological" acrosome reaction, sperm head presenting swelling of acrosomal ground substance with vesicles of fused plasma and outer acrosomal membranes. (C) Atypical acrosome reaction, sperm head presenting swelling of acrosomal ground substance dispersed under the swollen outer acrosomal membrane. (D) Damage in the acrosome, sperm head presenting rupture of the acrosomal membrane with swelling of acrosomal ground substance in restricted points. A: ×85,000; B~D: ×50,000. |
Acknowledgments
We would like to thank Biomedical Sciences Institute (ICBIM), Universidade Federal de Uberlandia (UFU), Uberlandia, Brazil.
References
1. Aitken RJ, Clarkson JS. Significance of reactive oxygen species and antioxidants in defining the efficacy of sperm preparation techniques. J Androl. 1988. 9:367–376.

2. Arcidiacono A, Walt H, Campana A, Balerna M. The use of Percoll gradients for the preparation of subpopulations of human spermatozoa. Int J Androl. 1983. 6:433–445.

3. Celeghini ECC, de Arruda RP, de Andrade AFC, Nascimento J, Raphael CF. Practical techniques for bovine sperm simultaneous fluorimetric assessment of plasma, acrosomal and mitochondrial membranes. Reprod Domest Anim. 2007. 42:479–488.

4. Cesari A, Kaiser GG, Mucci N, Mutto A, Vincenti A, Fornés MW, Alberio RH. Integrated morphophysiological assessment of two methods for sperm selection in bovine embryo production in vitro. Theriogenology. 2006. 66:1185–1193.

5. Cross NL, Meizel S. Methods for evaluating the acrosomal status of mammalian sperm. Biol Reprod. 1989. 41:635–641.
6. Cross NL, Morales P, Overstreet JW, Hanson FW. Two simple methods for detecting acrosome-reacted human sperm. Gamete Res. 1986. 15:213–226.

7. Dode MAN, Rodovalho NC, Ueno VG, Fernandes CE. The effect of sperm preparation and co-incubation time on in vitro fertilization of Bos indicus oocytes. Anim Reprod Sci. 2002. 69:15–23.

8. Flesch FM, Gadella BM. Dynamics of the mammalian sperm plasma membrane in the process of fertilization. Biochim Biophys Acta. 2000. 1469:197–235.

10. Le Lannou D, Blanchard Y. Nuclear maturity and morphology of human spermatozoa selected by Percoll density gradient centrifugation or swim-up procedure. J Reprod Fertil. 1988. 84:551–556.

11. Lu KH, Seidel GE Jr. Effects of heparin and sperm concentration on cleavage and blastocyst development rates of bovine oocytes inseminated with flow cytometrically-sorted sperm. Theriogenology. 2004. 62:819–830.

12. Machado GM, Carvalho JO, Filho ES, Caixeta ES, Franco MM, Rumpf R, Dode MAN. Effect of Percoll volume, duration and force of centrifugation, on in vitro production and sex ratio of bovine embryos. Theriogenology. 2009. 71:1289–1297.

13. McCann CT, Chantler E. Properties of sperm separated using Percoll and IxaPrep density gradients. A comparison made using CASA, longevity, morphology and the acrosome reaction. Int J Androl. 2000. 23:205–209.

14. Mehmood A, Anwar M, Saqlan Naqvi SM. Motility, acrosome integrity, membrane integrity and oocyte cleavage rate of sperm separated by swim-up or Percoll gradient method from frozen-thawed buffalo semen. Anim Reprod Sci. 2009. 111:141–148.

15. Morrell JM, Rodriguez-Martinez H. Biomimetic techniques for improving sperm quality in animal breeding: a review. Open Androl J. 2009. 1:1–9.
16. Oliveira LZ, Arruda RP, Celeghini ECC, de Andrade AFC, Perini AP, Resende MV, Miguel MCV, Lucio AC, Hossepian de Lima VFM. Effects of discontinuous Percoll gradient centrifugation on the quality of bovine spermatozoa evaluated with computer-assisted semen analysis and fluorescent probes association. Andrologia. 2011. doi: 10.1111/j.1439-0272.2010.01096.x. [Epub ahead of print].

17. Oshio S. Apparent densities of spermatozoa of various mammalian species. Gamete Res. 1988. 20:159–164.

18. Öura C, Toshimori K. Ultrastructural studies on the fertilization of mammalian gametes. Int Rev Cytol. 1990. 122:105–151.
19. Petyim S, Choavaratana R, Suksompong S, Laokirkkiat P, Makemaharn O. Outcome of sperm preparation using double-gradients technique study in siriraj hospital. J Med Assoc Thai. 2009. 92:878–884.
20. Rhemrev J, Jeyendran RS, Vermeiden JP, Zaneveld LJ. Human sperm selection by glass wool filtration and two-layer, discontinuous Percoll gradient centrifugation. Fertil Steril. 1989. 51:685–690.

21. Saacke RG, Marshall CE. Observations on the acrosomal cap of fixed and unfixed bovine spermatozoa. J Reprod Fertil. 1968. 16:511–514.

22. Somfai T, Bodó S, Nagy S, Papp ÁB, Iváncsics J, Baranyai B, Gócza E, Kovács A. Effect of Swim up and Percoll treatment on viability and acrosome integrity of frozen-thawed bull spermatozoa. Reprod Domest Anim. 2002. 37:285–290.

23. Sterzik K, De Santo M, Uhlich S, Gagsteiger F, Strehler E. Glass wool filtration leads to a higher percentage of spermatozoa with intact acrosomes: an ultrastructural analysis. Hum Reprod. 1998. 13:2506–2511.

24. Strehler E, Baccetti B, Sterzik K, Capitani S, Collodel G, De Santo M, Gambera L, Piomboni P. Detrimental effects of polyvinylpyrrolidone on the ultrastructure of spermatozoa (Notulae seminologicae 13). Hum Reprod. 1998. 13:120–123.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download