Abstract
Due primarily to the increasing shortage of allogeneic donor organs, xenotransplantation has become the focus of a growing field of research. Currently, micropigs are the most suitable donor animal for humans. However, no standard method has been developed to evaluate the systemic vascular anatomy of micropigs and standard reference values to aid in the selection of normal healthy animals as potential organ donors are lacking. Using 64-channel multidetector row computed tomographic angiography (MDCTA), we evaluated morphological features of the major systemic vessels in micropigs and compared our results to published human data. The main vasculature of the animals was similar to that of humans, except for the iliac arterial system. However, diameters of the major systemic vessels were significantly different between micropigs and humans. Specifically, the diameter of the aortic arch, abdominal aorta, external iliac artery, and femoral artery, were measured as 1.50 ± 0.07 cm, 0.85 ± 0.06 cm, 0.52 ± 0.05 cm, and 0.48 ± 0.05 cm, respectively, in the micropigs. This MDCTA data for micropig major systemic vessels can be used as standard reference values for xenotransplantation studies. The use of 64-channel MDCTA enables accurate evaluation of the major systemic vasculature in micropigs.
Organ transplantation is considered the preferred solution for the treatment of terminal organ failure [28]. However, there has always been a serious shortage of suitable human donors [13]. The constant shortage of donor organs led to research into xenotransplantation, which was first reported in 1906 [14]. Pigs are currently considered the most appropriate source of organs for xenotransplantation to humans based on several advantages, including physiological/anatomical organ similarities, reproductive characteristics, the possibility of controlled breeding, and ethical considerations [1,3,42]. Furthermore, recently, research to avoid the rejection of grafted organs has involved the production of genetically-modified pigs such as the α 1,3-galactosyltransferase gene knock-out pig, expression of human complement regulatory proteins (CD46, CD55, and/or CD59), and reducing risk of endogenous porcine retrovirus infection [6,8,20,26,27,32]. To resolve existing hurdles prior to the clinical application of pig organs, an appropriate method for evaluating the vascular system of micropigs must be established and immunological barriers must be addressed.
To transplant micropig solid organs into humans, evaluation of the vascular system and anatomical comparisons are essential for selection of a suitable organ as well as to gather sufficient pre-clinical data [5,18]. Previously, the standard method for preoperative angiographic evaluation of the donor vascular system was conventional angiography, whose disadvantages include being invasive and time-consuming, as well as the fact that it requires the use of ionizing radiation and large amounts of contrast agents. In contrast, the multidetector row computed tomographic angiography (MDCTA) process using doses of nonionic contrast media and ionizing radiation exposure that are less than conventional angiography [38]. Furthermore, venography is needed to obtain additional information regarding the venous system prior to organ transplantation. With remarkable advancements in spatial and temporal resolution, MDCTA is now routinely performed to evaluate human donors for solid organ transplant. This technology has been confirmed as a valuable method that can provide a road map for surgical planning as well as to assist in donor selection [17,33]. In addition, MDCTA has several advantages over traditional angiography; it is less invasive and permits visualization of organ structures and possible pathology [38].
The goal of this study was to confirm the feasibility of using MDCTA to evaluate the vascular system of micropigs and establish standard reference values for the vascular diameter and anatomy, which would be useful for selection of suitable donor organs in the future.
All experimental protocols were approved by the Ethics Committee of Chonnam National University, Korea (CNU IACUC-YB-2008-29). Physiologically and genetically intact male micropigs (n = 6) were purchased from PWG Genetics Korea (Korea). The animals were kept in individual cages at the university's central animal facility and received a standard pig diet and water ad libitum. The mean age and weight of the animals was 360 days and 30.50 ± 1.24 kg, respectively. Prior to undergoing MDCTA, all animals were fasted for 24 h. The animals were premedicated with an intramuscular injection of azaperone (0.5 mg/kg) and xylazine (8 mg/kg) and anesthetized with an intramuscular injection of a combination of zolazepam/tiletamine (4.4 mg/kg).
The examinations were performed using a 64-channel multi-detector row helical CT scanner (LightSpeed VCT; GE Healthcare, USA) according to the following parameters: 0.5 sec per rotation, 5 mm collimation, 1.0 pitch, and a tube current of 120 kV per 140~200 milliamperes. The MDCTA images were acquired with spatial resolution of 0.35 × 0.35 × 0.8 mm. The CT angiographic scan was obtained in the craniocaudal direction, and reconstruction thickness and reconstruction increment were 1 mm and 0.5 mm, respectively.
For administration of intravenous contrast material, a 20-gauge peripheral line was placed in an ear vein. After a scout CT image was obtained, arterial phase volumetric image data sets were acquired following initiation of an intravenous injection of 60 mL of nonionic contrast media (Ultravist 370; Schering AG, Germany) at the rate of 3 mL/sec using an automated injector (LF CT 9000; Liebel-Flarsheim, USA). An automatic bolus triggering software program was systematically applied, with a circular region of interest positioned at the level of the superior vena cava (SVC) and a threshold for triggering data acquisition preset at 100 Hounsfield units to obtain arterial phase images. All image acquisitions were obtained in the craniocaudal direction and supine position. Imaging extended from the C1 cervical vertebrae to the knee joint including both pelvis and thigh. Volumetric data sets were transferred to an Advantage Workstation 4.3 (GE Healthcare, USA) equipped with Volume Viewer Plus three-dimensional (3D) software for subsequent review. Transverse 0.625-mm-thick sections were reformatted into maximum intensity projection images and volume rendered images.
A single radiologist reviewed all CT images at a workstation which permitted editing of CT volume data sets to create optimal 3D CTA images. Source images as well as 3D display images were evaluated. For 3D CTA, volume-rendering techniques were typically employed, but maximum-intensity-projection rendering was also used as an adjunct display. The 3D images were reviewed by scrolling the acquisition displayed on a workstation monitor in conjunction with the assessment of conventional 2D axial images.
The reviewer measured and recorded the diameter of the aorta and major branches. The aorta was divided into four sections: ascending, arch, thoracic, and abdominal. Major aortic branches measured were right and left common carotids, celiac trunk, superior mesenteric, splenic, external iliac, and superficial femoral. The diameter of the main arteries was assessed from the most appropriate point of the segment, 1~1.5 cm from the ostium, using the workstation electronic cursor. The presence of any anatomic variations or intrinsic vascular disease such as atherosclerosis and/or calcification was also recorded.
In addition, both a morphological evaluation and measurement of the diameter of the SVC and inferior vena cava (IVC) were also performed. The diameter of the SVC was measured at the point just proximal to the SVC-right atrium junction. The diameter of the IVC was measured at three segments: hepatic, suprarenal, and infrarenal. The values presented in this study are expressed as mean ± SD. The data obtained from the micropigs was compared to pertinent human data published in the literature.
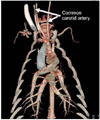
CT examinations were successfully performed in all six micropigs. There was no evidence of vascular malformation, arterial stenosis, aneurysm, atherosclerosis, or calcification found in any animal. In the present study, we measured the diameters of the major systemic vessels and compared those data to previously published human data (Table 1). The mean diameters of the right and left common carotid arteries measured were 0.57 ± 0.08 cm and 0.55 ± 0.05 cm, respectively (Fig. 1). There were no significant differences between micropigs and humans with regard to anatomy or diameter of the common carotid arteries.
The mean diameters of the micropig ascending and descending thoracic aorta, aortic arch, and SVC were 1.69 ± 0.12 cm, 1.23 ± 0.11 cm, 1.50 ± 0.07 cm, and 1.93 ± 0.33 cm, respectively. The anatomic structure of the thoracic aorta and aortic arch of the micropigs was similar to that of humans (Fig. 2), but the diameters of these vessels were considerably smaller than those in humans. In addition, the significant anatomical differences in SVC of micropig compared with human were not observed.
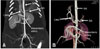
In the abdominal region, we evaluated the abdominal aorta, celiac trunk, superior mesenteric artery, splenic artery, and hepatic/suprarenal/infrarenal IVC. The mean diameters of these vessels were 0.85 ± 0.06 cm, 0.52 ± 0.08 cm, 0.68 ± 0.05 cm, 0.38 ± 0.05 cm, and 1.65 ± 0.20/1.59 ± 0.21/1.26 ± 0.07 cm, respectively. There were no anatomical variations in the micropigs in relation to humans; however, the diameter of the abdominal aorta was significantly smaller than in humans (Fig. 3). In addition, there were no significant differences between micropigs and humans with regards to anatomy or diameter of the IVC.
In the pelvic region, the diameters of the external iliac artery and superficial femoral artery were 0.52 ± 0.05 cm, and 0.48 ± 0.05 cm, respectively which were 42.4% and 46.3% comparable to human vessels, respectively.
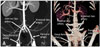
In all six micropigs examined, the external and internal iliac arteries arose directly from the aorta. There was no discernable common iliac artery in the micropigs. These findings were clearly different from the human vasculature (Fig. 4).
Solid-organ transplantation is currently the definitive solution for end-stage organ failure. Accurate preoperative imaging of donor vasculature is of great importance because vascular variations, such as accessory arteries and early branching, are particularly important when determining optimal organ extraction procedures and the type of anastomosis [7,24,37]. Furthermore, imaging evaluation of vascular systems using MDCTA plays a critical role in solid-organ transplantation to facilitate the selection of suitable donors, planning the surgical procedure, and revealing any co-existing pathology [17,33]. The gold standard technique for preoperative donor evaluation is conventional angiography, but this procedure has the drawback of being invasive [4]. Angiography using MDCT is fast, safe, minimally invasive, and now is routinely used in the preoperative evaluation of potential human donors for renal and liver transplantation [12,35,40,41]. In this study, we performed anatomical evaluations and diameter measurements of the major systemic vessels in micropigs using 64-channel MDCTA. The morphology and branching patterns of the major vessels were constant between the micropigs and there were no anatomical variations found during this study. In addition, the morphology of the major micropig vessels did not reveal significant differences when compared to those of humans, except for in the case of the iliac artery. In all micropigs evaluated, the external and internal iliac arteries arose directly from the aorta. The external artery detached one branch, the deep femoral artery, which continued as the femoral artery. There was no common iliac artery corresponding to that of humans, which arises from the aorta and branches off into the external and internal iliac arteries. Although differences in vascular diameter, morphology, and branching pattern between micropigs and human [2,19,25,30,39] can be overcome with modern surgical techniques at the time of transplantation, there is the possibility that the function of the related micropig organs could be compromised in human systems following transplantation. Thus, further studies are needed to evaluate and compare micropig organ function with that of humans.
In addition, the smaller diameter of micropig arteries compared to human vessels [11,16,31] may be problematic in terms of perioperative complications. It has been suggested that a smaller diameter donor artery may contribute to an increased incidence of post-transplantation complications. For example, hepatic arteries with diameters less than 3 mm are considered to present a high surgical risk for liver transplantation [15]; thus, accurate preoperative evaluation of the arterial diameter is essential for successful organ transplantation. Previous studies reported that CTA can replace conventional angiography traditionally used for preoperative evaluation of potential organ donors [4,21,22]. Along with the rapid evolution in technique, the number of detectors has gradually increased, allowing shorter scan rotation times, submillimeter slice acquisition parameters, and isotropic datasets [9,17,18,30]. MDCTA appears to be an ideal method to evaluate hepatic arteries and venous anatomy, as well as detect potential hepatic transplant complications such as hepatic artery and/or portal vein stenosis or thrombosis [10]. In addition, MDCTA has been reported to be as accurate as renal angiography for evaluating the arterial anatomy [29,34] and more sensitive for detecting venous and parenchymal structures [23]. Therefore, MDCTA is a suitable method to evaluate the anatomy of vascular structures of potential xenotransplantation donors as well as human recipients.
In conclusion, we present CTA data for the major systemic vessels in micropigs, which can be used as standard reference values for xenotransplantation studies. We have determined that 64-channel MDCTA allows accurate evaluation of the major systemic vasculature in micropigs.
Figures and Tables
Fig. 2
Coronal maximum intensity projection showing the normal structure of the aortic arch including the ascending/descending thoracic aorta.
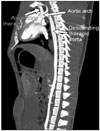
Fig. 3
Post-anterior views of the coronal maximum intensity projection (A) and the volume-rendered image (B) showing abdominal artery in the micropig. AA: abdominal aorta, CHA: common hepatic artery, SA: splenic artery.
Acknowledgments
This work was supported by a grant (code # 2007040-1034006) from the BioGreen 21 Program of the Rural Development Administration, Korea. The authors would like to acknowledge a graduate fellowship provided by the Ministry of Education and Human Resources Development through the Brain Korea 21 Project in Korea.
References
1. Appel JZ 3rd, Buhler L, Cooper DKC. The pig as a source of cardiac xenografts. J Card Surg. 2001. 16:345–356.

2. Ciccone MM, Favale S, Bhuva A, Scicchitano P, Caragnano V, Lavopa C, De Pergola G, Loverro G. Anteroposterior diameter of the infrarenal abdominal aorta is higher in women with polycystic ovary syndrome. Vasc Health Risk Manag. 2009. 5:561–566.
3. Cooper DKC, Gollackner B, Sachs DH. Will the pig solve the transplantation backlog? Annu Rev Med. 2002. 53:133–147.

4. Coşkun M, Kayahan EM, Özbek O, Çakır B, Dalgıç A, Haberal M. Imaging of hepatic arterial anatomy for depicting vascular variations in living related liver transplant donor candidates with multidetector computed tomography: comparison with conventional angiography. Transplant Proc. 2005. 37:1070–1073.

5. Cox A, Zhong R. Current advances in xenotransplantation. Hepatobiliary Pancreat Dis Int. 2005. 4:490–494.
6. Diamond LE, Quinn CM, Martin MJ, Lawson J, Platt JL, Logan JS. A human CD46 transgenic pig model system for the study of discordant xenotransplantation. Transplantation. 2001. 71:132–142.

7. Duong PA, Ferson PF, Fuhrman CR, McCurry KR, Lacomis JM. 3D-multidetector CT angiography in the evaluation of potential donors for living donor lung transplantation. J Thorac Imaging. 2005. 20:17–23.

8. Ezzelarab M, Cooper DK. Reducing Gal expression on the pig organ-a retrospective review. Xenotransplantation. 2005. 12:278–285.

9. Foley WD. Special focus session: multidetector CT: abdominal visceral imaging. Radiographics. 2002. 22:701–719.
10. Güven K, Acunaş B. Multidetector computed tomography angiography of the abdomen. Eur J Radiol. 2004. 52:44–55.

11. Hager A, Kaemmerer H, Rapp-Bernhardt U, Blücher S, Rapp K, Bernhardt TM, Galanski M, Hess J. Diameters of the thoracic aorta throughout life as measured with helical computed tomography. J Thorac Cardiovasc Surg. 2002. 123:1060–1066.

12. Hiroshige S, Shimada M, Harada N, Shiotani S, Ninomiya M, Minagawa R, Soejima Y, Suehiro T, Honda H, Hashizume M, Sugimachi K. Accurate preoperative estimation of liver-graft volumetry using three-dimensional computed tomography. Transplantation. 2003. 75:1561–1564.

13. Hosenpud JD, Bennett LE, Keck BM, Fiol B, Boucek MM, Novick RJ. The Registry of the International Society for Heart and Lung Transplantation: fifteenth official report-1998. J Heart Lung Transplant. 1998. 17:656–668.

14. Hunter P. Xeno's paradox. Why pig cells are better for tissue transplants than human cells. EMBO Rep. 2009. 10:554–557.
15. Inomoto T, Nishizawa F, Sasaki H, Terajima H, Shirakata Y, Miyamoto S, Nagata I, Fujimoto M, Moriyasu F, Tanaka K, Yamaoka Y. Experiences of 120 microsurgical reconstructions of hepatic artery in living related liver transplantation. Surgery. 1996. 119:20–26.

16. Kahraman H, Ozaydin M, Varol E, Aslan SM, Dogan A, Altinbas A, Demir M, Gedikli O, Acar G, Ergene O. The diameters of the aorta and its major branches in patients with isolated coronary artery ectasia. Tex Heart Inst J. 2006. 33:463–468.
17. Kamel IR, Kruskal JB, Warmbrand G, Goldberg SN, Pomfret EA, Raptopoulos V. Accuracy of volumetric measurements after virtual right hepatectomy in potential donors undergoing living adult liver transplantation. AJR Am J Roentgenol. 2001. 176:483–487.

18. Kawamoto S, Fishman EK. MDCT angiography of living laparoscopic renal donors. Abdom Imaging. 2006. 31:361–373.

19. Krejza J, Arkuszewski M, Kasner SE, Weigele J, Ustymowicz A, Hurst RW, Cucchiara BL, Messe SR. Carotid artery diameter in men and women and the relation to body and neck size. Stroke. 2006. 37:1103–1105.

20. Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, Samuel M, Bonk A, Rieke A, Day BN, Murphy CN, Carter DB, Hawley RJ, Prather RS. Production of α-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002. 295:1089–1092.

21. Lee MW, Lee JM, Lee JY, Kim SH, Park EA, Han JK, Kim YJ, Shin KS, Suh KS, Choi BI. Preoperative evaluation of the hepatic vascular anatomy in living liver donors: comparison of CT angiography and MR angiography. J Magn Reson Imaging. 2006. 24:1081–1087.

22. Lee SS, Kim TK, Byun JH, Ha HK, Kim PN, Kim AY, Lee SG, Lee MG. Hepatic arteries in potential donors for living related liver transplantation: evaluation with multi-detector row CT angiography. Radiology. 2003. 227:391–399.

23. Lerner LB, Henriques HF, Harris RD. Interactive 3-dimensional computerized tomography reconstruction in evaluation of the living renal donor. J Urol. 1999. 161:403–407.

24. Lin CH, Steinberg AP, Ramani AP, Abreu SC, Desai MM, Kaouk J, Goldfarb DA, Gill IS. Laparoscopic live donor nephrectomy in the presence of circumaortic or retroaortic left renal vein. J Urol. 2004. 171:44–46.

25. Machálek L, Holibková A, Tůma J, Houserková D. The size of the splenic hilus, diameter of the splenic artery and its branches in the human spleen. Acta Univ Palacki Olomuc Fac Med. 1998. 141:45–48.
26. Murakami H, Nagashima H, Takahagi Y, Miyagawa S, Fujimura T, Toyomura K, Nakai R, Yamada M, Kurihara T, Shigehisa T, Okabe M, Seya T, Shirakura R, Kinoshita T. Transgenic pigs expressing human decay-accelerating factor regulated by porcine MCP gene promoter. Mol Reprod Dev. 2002. 61:302–311.

27. Niemann H, Verhoeyen E, Wonigeit K, Lorenz R, Hecker J, Schwinzer R, Hauser H, Kues WA, Halter R, Lemme E, Herrmann D, Winkler M, Wirth D, Paul D. Cytomegalovirus early promoter induced expression of hCD59 in porcine organs provides protection against hyperacute rejection. Transplantation. 2001. 72:1898–1906.

28. Ogata K, Platt JL. Cardiac xenotransplantation: future and limitations. Cardiology. 2004. 101:144–155.

29. Platt JF, Ellis JH, Korobkin M, Reige KA, Konnak JW, Leichtman AB. Potential renal donors: comparison of conventional imaging with helical CT. Radiology. 1996. 198:419–423.

31. Rådegran G, Saltin B. Human femoral artery diameter in relation to knee extensor muscle mass, peak blood flow, and oxygen uptake. Am J Physiol Heart Circ Physiol. 2000. 278:H162–H167.
32. Ramsoondar JJ, Máchaty Z, Costa C, Williams BL, Fodor WL, Bondioli KR. Production of α1,3-galactosyltransferase-knockout cloned pigs expressing human a α1,2-fucosylosyltransferase. Biol Reprod. 2003. 69:437–445.

33. Rankin SC, Jan W, Koffman CG. Noninvasive imaging of living related kidney donors: evaluation with CT angiography and gadolinium-enhanced MR angiography. AJR Am J Roentgenol. 2001. 177:349–355.
34. Rubin GD, Alfrey EJ, Dake MD, Semba CP, Sommer FG, Kuo PC, Dafoe DC, Waskerwitz JA, Bloch DA, Jeffrey RB. Assessment of living renal donors with spiral CT. Radiology. 1995. 195:457–462.

35. Schroeder T, Nadalin S, Stattaus J, Debatin JF, Malagó M, Ruehm SG. Potential living liver donors: evaluation with an all-in-one protocol with multi-detector row CT. Radiology. 2002. 224:586–591.

36. Silveira LA, Silveira FB, Fazan VP. Arterial diameter of the celiac trunk and its branches. Anatomical study. Acta Cir Bras. 2009. 24:43–47.

37. Smith PA, Ratner LE, Lynch FC, Corl FM, Fishman EK. Role of CT angiography in the preoperative evaluation for laparoscopic nephrectomy. Radiographics. 1998. 18:589–601.

38. Tombul ST, Aki FT, Gunay M, Inci K, Hazirolan T, Karcaaltincaba M, Erkan I, Bakkaloglu A, Yasavul U, Bakkaloglu M. Preoperative evaluation of hilar vessel anatomy with 3-D computerized tomography in living kidney donors. Transplant Proc. 2008. 40:47–49.

39. Toprak A, Koc M, Tezcan H, Ozener IC, Akoglu E, Oktay A. Inferior vena cava diameter determines left ventricular geometry in continuous ambulatory peritoneal dialysis patients: an echocardiographic study. Nephrol Dial Transplant. 2003. 18:2128–2133.

41. Valastro M, Veroux M, Macarone M, Cappello D, Vizcarra D, Gagliano M, Di Mare M, Spataro M, Giuffrida G, Tallarita T, Magnano San Lio V, Veroux P. Multi-detector row CT scanner angiography in the evaluation of living kidney donors. Chir Ital. 2007. 59:337–341.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download