Abstract
A simplified polymerase chain reaction (PCR) assay was developed for fast and easy screening of mycoplasma mastitis in dairy cattle. Species of major mycoplasma strains [Mycoplasma (M.) bovis, M. arginini, M. bovigenitalium, M. californicum, M. bovirhinis, M. alkalescens and M. canadense] in cultured milk samples were detected by this simplified PCR-based method as well as a standard PCR technique. The minimum concentration limit for detecting mycoplasma by the simplified PCR was estimated to be about 2.5 × 103 cfu/mL and was similar to that of the standard PCR. We compared the specificity and sensitivity of the simplified PCR to those of a culture method. Out of 1,685 milk samples cultured in mycoplasma broth, the simplified PCR detected Mycoplasma DNA in 152 that were also positive according to the culture assay. The sensitivity and specificity of the simplified PCR were 98.7% and 99.7%, respectively, for detecting mycoplasma in those cultures. The results obtained by the simplified PCR were consistent with ones from standard PCR. This newly developed simplified PCR, which does not require DNA purification, can analyze about 300 cultured samples within 3 h. The results from our study suggest that the simplified PCR can be used for mycoplasma mastitis screening in large-scale dairy farms.
Mycoplasma are highly contagious pathogens and intramammary infection by these species is a serious problem in dairy herds [7]. Clinical cases of mycoplasma mastitis exhibit severe clinical symptoms including fever along with swelling and induration of the udder [3]. Abnormal milk with flaky sediments in watery or serous fluid is also observed in the infected quarter. Since the cure rate of clinical mastitis caused by Mycoplasma spp. is very low because of their resistance to antibiotic therapy, Mycoplasma-infected cows must be culled in an emergency to prevent an outbreak of mycoplasma mastitis in dairy herds [5,6,8]. Polymerase chain reaction (PCR) is an accurate method for diagnosing Mycoplasma infection [2,4,9,10]. PCR has been widely accepted as a reliable method for detecting mycoplasma strains in milk samples. However, DNA extraction from a large number of milk samples is labor-intensive for laboratories in veterinary hospitals. Fast and easy screening for mycoplasma mastitis using a simplified PCR would make it possible to isolate infected cows from herds and prevent outbreaks mycoplasma mastitis on commercial dairy farms. This study describes the development of a simplified PCR assay for fast and easy mycoplasma mastitis screening on commercial dairy farms.
The following bacteria strains were used: Mycoplasma (M.) bovis (ATCC 25523), M. arginini (ATCC 23838), M. bovigenitalium (ATCC 19852), M. californicum (ATCC 33461), M. bovirhinis (ATCC 27748), M. alkalescens (ATCC 29103), and M. canadense (ATCC 29418). Additionally, each species of two Mycoplasma strains isolated from cases of naturally occurring mycoplasma mastitis were used for this study. All strains were grown in mycoplasma culture broth (Kanto Kagaku, Japan) at 37℃ for 72 h.
Simplified PCR was performed in a total reaction volume of 20 µL containing 10 µL of 2 × Ampdirect Plus (Shimadzu, Japan), 0.5 U of Nova taq TM Hot Start DNA polymerase (Novagen, UK), 5 pmol of a mycoplasma universal primer set (MycoAce; Nihon Dobutsu Tokusyu Shindan, Japan), and 5 µL of each mycoplasma suspension (103, 104, and 105 cfu/mL), which were equal to 2.5 × 102, 2.5 × 103, and 2.5 × 104 cfu/mL. The DNA extraction and purification is unnecessary for this PCR method. PCR was performed in an iCycler PCR System (Biorad, USA). Conditions for the simplified PCR were as follows: initial denaturation at 95℃ for 10 min followed by 35 cycles of denaturation at 94℃ for 30 sec, annealing at 60℃ for 45 sec, and extension at 72℃ for 1 min. The PCR products were separated by electrophoresis on 1.5% (w/v) agarose gels, stained with ethidium bromide, and visualized with a UV transilluminator.
To compare the performance of the simplified PCR with that of standard PCR, we performed a standard PCR assay as previously described [2]. Briefly, DNA from a Mycoplasma suspension was extracted using a DNA extraction kit (Qiagen, Germany) according to the manufacturer's instructions. Standard PCR was performed in a total reaction volume of 20 µL containing 10 × buffer (GE Healthcare, UK), 0.5 U Taq DNA polymerase (GE Healthcare, UK), 4 mM dNTPs, 5 pmol mycoplasma universal primer set, and 5 µL of DNA template which was equal to 2.5 × 102, 2.5 × 103, and 2.5 × 104 cfu/mL of Mycoplasma. Conditions of the PCR and electrophoresis were the same as those for the simplified PCR described above.
To evaluate the usefulness of the simplified PCR on commercial dairy farms, we compared the sensitivity and specificity of the simplified PCR to that of a culture method. A total of 1,683 quarter milk samples from lactating cows were randomly collected from 18 commercial dairy farms. A total of 159 milk samples were collected from quarters with clinical symptoms such as swelling, induration, and flare. We confirmed that 202 milk samples were collected from quarters that showed no clinical symptoms but had high somatic cell counts (> 400 × 103 cfu/mL). One hundred µL of milk sample were used to inoculate 2.9 mL of mycoplasma broth (Kanto Kagaku, Japan) and incubated at 37℃ for 72 h. One hundred µL of the broth culture were then plated on a Mycoplasma agar plate (Kanto Kagaku, Japan) and incubated in 5% CO2 at 37℃ for 14 to 28 days to produce typical Mycoplasma colonies [7]. Each broth culture was analyzed using the simplified and standard PCR as described above.
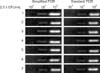
In this study, we compared the detection rates for seven major Mycoplasma spp. of the simplified and standard PCR assays. All ATCC strains were clearly detected by both methods (Fig. 1). Seven species of two mycoplasma strains isolated from animals with mycoplasma mastitis were also detected by both simplified and standard PCR (data not shown). Gene sequences of PCR-amplified products showed 99% homology with documented sequences in an established gene bank (intergenic spacer region). The minimum detection limit for Mycoplasma by simplified PCR was estimated to be 2.5 × 103 cfu/mL (Fig. 2). The results obtained from the simplified PCR agreed well with those from standard PCR using purified DNA from broth cultures. Our results showed that simplified PCR for detecting Mycoplasma spp. permits gene amplification without any DNA preparation and performs as well as standard PCR. In this study, we used Amdirect Plus for the simplified PCR to detect Mycoplasma spp. in broth cultures. Ampdirect Plus is a commercially available reagent used for preparing PCR samples without DNA extraction and purification [1]. We have confirmed that the use of Ampdirect Plus reduces labor and time for detecting Mycoplasma spp. in samples.
Out of the 1,685 milk samples cultured in mycoplasma broth, simplified PCR detected Mycoplasma DNA in 152 samples that were also positive according to the culture assay. The concentration of Mycoplasma in these broth cultures was more than 1 × 104 cfu/mL. Four samples were found to be negative by culture and positive by simplified PCR. We speculated that the amplification of DNA from non-viable Mycoplasma in the broth cultures caused the differences between the simplified PCR and culture method results. Two samples were found to be negative by the simplified PCR and positive by culture assay. We confirmed that the concentrations of Mycoplasma in these cultured broths were 2 × 102 and 3 × 102 cfu/mL, which are less than the minimum detection limit of the simplified PCR. Both milk samples were obtained from cows with no clinical symptoms and normal somatic cell counts. Our results suggested that the number of Mycoplasma in the broth cultures of a few milk samples was insufficient for simplified PCR. These samples were not further investigated. The sensitivity and specificity of the simplified PCR method were 98.7% and 99.7%, respectively, of those of the culture assay. Results from the simplified PCR assay completely concurred with those obtained by standard PCR. It has been reported that the sensitivity and specificity of standard PCR for detecting Mycoplasma are 96.2% and 99.1%, respectively, of those of the culture method [2]. In the present study, we succeeded in establishing a simplified PCR method that is able to provide the results identical to those obtained using conventional culture and standard PCR methods.
Standard PCR techniques are labor-intensive and time-consuming, making this method impractical for assaying a large number of milk samples from a commercial dairy farm. This method requires many steps that are quite lengthy; processing about 300 samples requires at least 4~5 h for DNA template preparation and 3 h for completing the PCR cycle. In contrast, 300 samples to be examined within 3 h using the simplified PCR, which does not require several of these laborious steps including Mycoplasma DNA template isolation and purification. Fast and easy screening for mycoplasma mastitis using a simplified PCR assay would enable the quick isolation of infected cows from herds. Our newly developed simplified PCR assay for detecting Mycoplasma spp. is a useful method that can be used to help control and prevent mycoplasma mastitis outbreaks on commercial dairy farms.
Figures and Tables
Fig. 1
Detection of major mycoplasma mastitis pathogens by simplified (upper) and standard (lower) PCR. M. marker, 1: Mycoplasma (M.) bovis (ATCC 25523), 2: M. arginini (ATCC 23838), 3: M. bovigenitalium (ATCC 19852), 4: M. californicum (ATCC 33461), 5: M. bovirhinis (ATCC 27748), 6: M. alkalescens (ATCC 29103), 7: M. canadense (ATCC 29418).
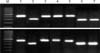
Fig. 2
Minimum limits of detection for major mycoplasma mastitis pathogens by simplified PCR and standard PCR. 1: M. bovis (ATCC 25523), 2: M. arginini (ATCC 23838), 3: M. bovigenitalium (ATCC 19852), 4: M. californicum (ATCC 33461), 5: M. bovirhinis (ATCC 27748), 6: M. alkalescens (ATCC 29103), 7: M. canadense (ATCC 29418).
Acknowledgments
Our study was supported in part by the Support Project to Assist Private Universities in Developing Bases for Research from Ministry of Education, Culture, Sports, Science and Technology, and by Akiyama Life Science Foundation, Japan.
References
1. Alshahni MM, Makimura K, Yamada T, Satoh K, Ishihara Y, Takatori K, Sawada T. Direct colony PCR of several medically important fungi using Ampdirect plus. Jpn J Infect Dis. 2009. 62:164–167.
2. Baird SC, Carman J, Dinsmore RP, Walker RL, Collins JK. Detection and identification of Mycoplasma from bovine mastitis infections using a nested polymerase chain reaction. J Vet Diagn Invest. 1999. 11:432–435.

4. Ghadersohi A, Coelen RJ, Hirst RG. Development of a specific DNA probe and PCR for the detection of Mycoplasma bovis. Vet Microbiol. 1997. 56:87–98.

5. Jasper DE. The role of Mycoplasma in bovine mastitis. J Am Vet Med Assoc. 1982. 181:158–162.
6. Kirk JH, Lauerman LH. Mycoplasma mastitis in dairy cows. Compend Contin Educ Pract Vet. 1994. 16:541–551.
7. Nicholas RAJ, Ayling RD. Mycoplasma bovis: disease, diagnosis, and control. Res Vet Sci. 2003. 74:105–112.
8. Pfützner H, Sachse K. Mycoplasma bovis as an agent of mastitis, pneumonia, arthritis and genital disorders in cattle. Rev Sci Tech. 1996. 15:1477–1494.
9. Riffon R, Sayasith K, Khalil H, Dubreuil P, Drolet M, Lagacé J. Development of a rapid and sensitive test for identification of major pathogens in bovine mastitis by PCR. J Clin Microbiol. 2001. 39:2584–2589.

10. Sung H, Kang SH, Bae YJ, Hong JT, Chung YB, Lee CK, Song S. PCR-based detection of Mycoplasma species. J Microbiol. 2006. 44:42–49.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download