Abstract
We investigated the immunostimulatory effects of a novel β-glucan purified from Paenibacillus (P.) polymyxa JB115 on bone marrow-derived dendritic cells (DCs), a type of potent antigen-presenting cells. β-glucan isolated from P. polymyxa JB115 enhanced the viability and induced the maturation of DCs. β-glucan markedly increased the cytokine production of DCs and surface expression of DC markers. In addition, DCs treated with β-glucan showed a higher capacity to stimulate allogeneic spleen cell proliferation compared to those treated with medium alone. These results demonstrate the effect of β-glucan on DC maturation and may increase the use of β-glucan.
β-glucans are heterogeneous glucose polymers that form a structural extracellular matrix in pathogenic microbes [1]. These compounds are recognized by several receptors on the surface of immune cells [4]. Recent studies demonstrated that a novel β-glucan purified from Paenibacillus (P.) polymyxa JB115 [8] induces macrophage activation [2].
Dendritic cells (DCs) are the most potent antigen-presenting cells in the immune system [12]. They are specialized for capturing and processing antigens, then presenting antigenic peptides to naïve T lymphocytes to initiate antigen-specific immune responses [13]. However, the biological effects of β-glucan purified from P. polymyxa JB115 on DCs remain unclear. In the present study, we evaluated the various biological effects of β-glucan on DCs.
To investigate the effects of β-glucan purified from P. polymyxa JB115 on DCs, we prepared immature DCs from bone marrow as previously described [10], and treated these cells with 1~100 µg/mL β-glucan. Two days after β-glucan treatment, clusters of DCs were observed using an inverted microscope connected to a digital camera (Fig. 1A). In addition, cell size was analyzed using flow cytometry. It was found that β-glucan enhanced the percentage of normal-sized DCs in the forward scatter/side scatter dot plot (Fig. 1B). A cell viability assay using a trypan blue solution demonstrated that 100 µg/mL β-glucan significantly increased the viability of DCs (Fig. 2). Thus, β-glucan may enhance the survival of DCs and also helps maintain normal cell size.
The production of tumor necrosis factor (TNF)-α and interleukin (IL)-12 by DCs treated with β-glucan was determined by an ELISA. IL-12 and TNF-α are representative cytokines involved in cell-mediated and innate immunity, respectively, along with DC survival [3,5]. β-glucan (100 µg/mL) significantly increased the production of both cytokines (Figs. 3A and B). In addition, β-glucan enhanced the release of nitric oxide from DCs (Fig. 3C). Nitric oxide plays a critical role in eliminating intracellular pathogens in macrophages.
To determine whether β-glucan induces the maturation of DCs, expression levels of immune-related DC surface markers were measured by flow cytometric analysis [9]. Major histocompatibility complex (MHC) class I and II are major antigen-presenting molecules on DCs while CD54 and CD86 are adhesion and co-stimulatory molecules, respectively [11]. β-glucan consistently enhanced the expression all of the surface markers investigated in the present study (Fig. 4A). Because MHC class II and CD86 molecules have been identified as maturation markers of bone marrow-derived DCs [6,12], β-glucan may induce the maturation of DCs. Decreased antigen uptake is a major characteristic of mature DCs. Antigen uptake analysis using dextran-fluorescein isothiocyanate revealed that β-glucan profoundly decreased the antigen uptake capability of DCs compared to immature DCs (Fig. 4B). Zymosan was used as a positive control in this assay.
To confirm the antigen-presenting capability of DCs treated with β-glucan, we prepared allogeneic splenocytes from BALB/c mice [7]. The cells were then stained with carboxyfluorescein succinimidyl ester (CFSE) and co-cultured with immature or β-glucan-treated DCs. Highly proliferative cells had a relatively low CFSE intensity detected as FL1 by flow cytometric analysis because of their high cell division rates. Splenocytes cocultured with DCs treated with β-glucan (100 µg/mL) showed the lowest CFSE intensity, indicating that these cells had the highest proliferative capacity (Fig. 5). These observations showed that β-glucan enhances the antigen-presenting capability of DCs. Taken together, our results demonstrated that β-glucan from P. polymyxa JB115 induces the maturation of DCs, the most potent antigen-presenting cells in host immunity. This study may provide researchers with valuable information to increase the use of β-glucan.
Figures and Tables
Fig. 1
Clusters of dendritic cells (DCs) treated with β-glucan. DCs were seeded at a density of 5 × 105 cells/mL in 6-well culture plates, and then treated with β-glucan for 2 days. (A) DC morphology. ×200. (B) The number and size of DCs was measured by flow cytometric analysis.
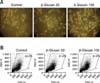
Fig. 2
Effect of β-glucan on DC viability. DCs were seeded at a concentration of 2.5 × 105 cells/mL in 96-well culture plates, and then treated with the indicated concentrations of β-glucan for 2 days. For the viability assay, treated cells were stained with a trypan blue staining solution to determine the number of live and dead cells. Data are presented as the mean ± SD from four individual wells. **p < 0.01.

Fig. 3
β-glucan increases cytokine and nitric oxide production of DCs. DCs were seeded and treated as described in Fig. 2. The amounts of interleukin (IL)-12 (A), tumor necrosis factor (TNF)-α (B), and nitric oxide (C) produced by DCs were measured. Data are presented as the mean ± SD from four individual wells. **p < 0.01, ***p < 0.001.

Fig. 4
β-glucan enhances the expression of immune-related DC surface markers but decreases DC antigen uptake. DCs were prepared and treated as described in the legend for Fig. 1. For surface marker analysis (A), the number in the histogram indicates the mean fluorescence intensity (MFI) of the main DC population. For antigen uptake analysis (B), DCs were incubated with 250 µg/mL dextran-fluorescein isothiocyanate at 4℃ or 37℃ for 45 min. The number in the histogram indicates the MFI of viable DCs.

Fig. 5
β-glucan increases the allo-proliferative capability of DCs. DC cultures were established and treated as described in the legend for Fig. 1. For the allo-proliferation assay, DCs treated with β-glucan (1 × 104 cells/well) were co-cultured for 5 days with allogeneic splenocytes (2 × 105 cells/well) stained with 5 µM carboxyfluorescein succinimidyl ester. The number indicates the percentage of proliferating cells with a low FL1 value in the co-culture.

Acknowledgments
This research was supported by Technology Development Program for Agriculture and Forestry, Ministry for Food, Agriculture, Forestry and Fisheries, Korea.
References
2. Chang ZQ, Lee JS, Gebru E, Hong JH, Jung HK, Jo WS, Park SC. Mechanism of macrophage activation induced by β-glucan produced from Paenibacillus polymyxa JB115. Biochem Biophys Res Commun. 2010. 391:1358–1362.

3. Esche C, Shurin GV, Kirkwood JM, Wang GQ, Rabinowich H, Pirtskhalaishvili G, Shurin MR. Tumor necrosis factor-α-promoted expression of Bcl-2 and inhibition of mitochondrial cytochrome c release mediate resistance of mature dendritic cells to melanoma-induced apoptosis. Clin Cancer Res. 2001. 7:3 Suppl. 974s–979s.
4. Goodridge HS, Wolf AJ, Underhill DM. β-glucan recognition by the innate immune system. Immunol Rev. 2009. 230:38–50.

5. Heufler C, Koch F, Stanzl U, Topar G, Wysocka M, Trinchieri G, Enk A, Steinman RM, Romani N, Schuler G. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-γ production by T helper 1 cells. Eur J Immunol. 1996. 26:659–668.

6. Inaba K, Witmer-Pack M, Inaba M, Hathcock KS, Sakuta H, Azuma M, Yagita H, Okumura K, Linsley PS, Ikehara S, Muramatsu S, Hodes RJ, Steinman RM. The tissue distribution of the B7-2 costimulator in mice: abundant expression on dendritic cells in situ and during maturation in vitro. J Exp Med. 1994. 180:1849–1860.

7. Joo HG, Goedegebuure PS, Sadanaga N, Nagoshi M, von Bernstorff W, Eberlein TJ. Expression and function of galectin-3, a β-galactoside-binding protein in activated T lymphocytes. J Leukoc Biol. 2001. 69:555–564.
8. Jung HK, Hong JH, Park SC, Park BK, Nam DH, Kim SD. Production and physicochemical characterization of β-glucan produced by Paenibacillus polymyxa JB115. Biotechnol Bioprocess Eng. 2007. 12:713–719.

9. Kim MH, Byon YY, Ko EJ, Song JY, Yun YS, Shin T, Joo HG. Immunomodulatory activity of ginsan, a polysaccharide of Panax ginseng, on dendritic cells. Korean J Physiol Pharmacol. 2009. 13:169–173.

10. Kim MH, Joo HG. Immunostimulatory effects of fucoidan on bone marrow-derived dendritic cells. Immunol Lett. 2008. 115:138–143.

11. Larsen CP, Ritchie SC, Hendrix R, Linsley PS, Hathcock KS, Hodes RJ, Lowry RP, Pearson TC. Regulation of immunostimulatory function and costimulatory molecule (B7-1 and B7-2) expression on murine dendritic cells. J Immunol. 1994. 152:5208–5219.
12. Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001. 106:255–258.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download