Abstract
The objective of this study was to evaluate and validate the accuracy of the Perkins handheld applanation tonometer for measuring intraocular pressure (IOP) in horses and cattle. Both eyes of 10 adult horses and cattle were evaluated in a postmortem study. The eyes from 10 clinically normal adult horses and cattle were also examined after bilateral auriculopalpebral nerve block and topical anesthesia for an in vivo study. IOP was measured postmortem using direct manometry (measured with an aneroid manometer) and tonometry (measured with a Perkins handheld applanation tonometer). The correlation coefficients (r2) for the data from the postmortem manometry and Perkins tonometer study were 0.866 for horses and 0.864 for cattle. In the in vivo study, IOP in horses was 25.1 ± 2.9 mmHg (range 19.0~30.0 mmHg) as measured by manometry and 23.4 ± 3.2 mmHg (range 18.6~28.4 mmHg) according to tonometry. In cattle, IOP was found to be 19.7 ± 1.2 mmHg (range 18.0~22.0 mmHg) by manometry and 18.8 ± 1.7 mmHg (range 15.9~20.8 mmHg) by tonometry. There was a strong correlation between the IOP values obtained by direct ocular manometry and the tonometer in both horses and cattle. Our results demonstrate that the Perkins handheld tonometer could be an additional tool for accurately measuring IOP in equine and bovine eyes.
Glaucoma is an optic neuropathy characterized by the death of retinal ganglion cells (RGC) which results in a loss of vision. Increased intraocular pressure (IOP) is the principal cause of RGC dysfunction with subsequent degeneration of the optic nerve [2,16,18,23]. IOP can be measured by either manometry or tonometry. Ocular manometry is considered to be the gold standard for determining IOP as this technique measures the actual IOP. However, this is an invasive procedure used experimentally and involves cannulation of the anterior chamber to measure IOP with either a digital instrument or a column of water or mercury [5,15,17,19,20].
Tonometry measures IOP using devices called tonometers which use different techniques to evaluate corneal tension induced by IOP [14,16,19]. Tonometers can be classified according to whether they come into contact with the cornea, if they are portable or not, and the method used for IOP measurement [14]. These methods include indentation, applanation, and, more recently, the rebound technique [10,11].
Applanation tonometry is based on the principle that the force required to flatten a certain area of a spherical surface is the same as the pressure inside that sphere [19]. The Goldman applanation tonometer was the first to be used in human medicine. Later, some handheld applanation tonometers appeared which allowed the examination of bedridden patients and children [4,19]. In veterinary medicine, portable contact applanation tonometers are most commonly used [14,16] among which the Tonopen XL is the most popular, especially in horse and cattle [2,7,18,23].
In equine ophthalmology, indentation tonometry with the Schiotz tonometer is not possible due to the thickness of the cornea and the inability to position the head of the awake animal horizontally [2,18,21]. Applanation tonometry therefore is essential for diagnosing equine glaucoma in most clinical circumstances [18].
The Perkins tonometer is a very popular handheld applanation tonometer used in humans. This device uses a Goldmann prism (3 mm double prism) that is adjusted during tonometry to form fluorescein semicircles from a small blue light source [4,19]. The tonometer has a scale ranging from 0 to 5 with subdivisions of 0.2. Thus, the large divisions of the scale represent grams and the small divisions 0.2 g, with examinations typically started with the scale at 1 [19]. In humans, readings measured on this scale are multiplied by 10 to determine the tension in mmHg [4,19]. In a recent study of dogs and cats [1], it is approximately the same, in other words, it is also multiplied by 10. While measurement accuracy depends on the experience of the operator, the Perkins tonometer is approximately 3 to 5 times cheaper than the Tonopen XL and Tonovet [1].
The studies using the Perkins tonometer have been conducted on dogs, cats, and rabbits [1,8,9,12]. However, use of this tonometer has still not been reported in horses, and there has only been one cattle study that used this tonometer [6]. Two rabbit studies have been conducted. One compared the effectiveness and accuracy of 3 tonometers, Tonopen XL, Perkins and ocular blood flow pneumatonometer [12]. The other compared ability of the the Perkins, Tonopen XL, and Tonovet tonometers to measure IOP. Both studies found that the Perkins tonometer had the highest degree of accuracy [9]. Therefore, the objective of this study was to evaluate and validate the accuracy of the Perkins handheld applanation tonometer for measuring IOP in horses and cattle.
This experiment was approved by the Ethical Committee (Protocol No. 150/07 and No. 161/07), University of Oeste Paulista (UNOESTE), Brazil. A pilot study previously authorized by the Ethical Committee was conducted to evaluate the use of invasive manometry in horses and cattle. The procedure was performed after bilateral auriculopalpebral nerve block and topical anesthesia had been administered, mainly due to the position the horizontal position of the head when the animals were awake and to measure actual IOP without the influence of general anesthesia. The procedure was well-tolerated by both horses and cows and deemed to be safe for performing manometry without causing discomfort or pain in the animals.
Twenty eyes from ten adult horses and cattle were obtained for the postmortem study from a slaughterhouse (King Meat) and Bom Mart (Brazil), respectively. Both eyes in all animals were left within the orbits, and the heads of the animals were obtained immediately after sacrifice and transported in refrigerated boxes. The experiment was performed within 1 h after sacrifice. For the in vivo study, 10 adult conscious horses and cattle were obtained from the Veterinary Teaching Farm of UNOESTE. Only normal eyes were used in this study as determined by ophthalmic examination (direct ophthalmoscopy, pupillary light reflex, Schirmer Tear Test, and fluorescein test). The ophthalmic exams and Perkins tonometry were always performed by the same observer to prevent inter-observer variability. After the procedure, the animals were returned to pasture at the UNOSTE Veterinary Teaching Farm.
For the postmortem study (Fig. 1), each eye was cannulated with a 23-gauge scalp vein needle (Embramac, Brazil) through the cornea about 1 to 2 mm from the limbus in the supero-lateral quadrant. Cyanoacrylate glue (Superbond; Loctite, Brazil) was applied around the needle to prevent leakage of the aqueous humor. The needle was connected via a polyethylene tube to a three-way stopcock. The stopcock was connected to a reservoir (syringe) containing 10 mL of a physiological saline solution (Fresenius Kabi, Brazil) and to an aneroid manometer (Missouri, Brazil), which was set at the zero position relative to the center of the eye. The calibration curve for manometry versus tonometry was determined by artificially increasing the IOP in 5 mmHg increments up to 50 mmHg in tetracaine 1% the open stopcock mode. Prior to taking the tonometer reading, one drop of 1% fluorescein (Allergan, Brazil) was administered to allow for the formation of fluorescein semicircles. Three readings were taken with the Perkins tonometer (Clement Clarke, UK) and the mean value was calculated.
For the in vivo study (Figs. 2 and 3), the horses were led into a solid-sided restraint cage with a bar in front and a bar or solid gate behind. If necessary, the horse's head was positioned above the level of the heart for measuring IOP with the Perkins tonometer and manometer by using a twitch on the upper lip. The procedures to measure IOP in the horses and cattle always started at 8.00 a.m. and ended at 10.00 a.m. The cattle were guided into a funnel corral and then restrained with a neck yoke. Bilateral auriculopalpebral nerve blocks with 10 mL of 2% lidocaine HCl (Hipolabor, Brazil) and bilateral topical anesthesia with two drops of tetracaine 1% (Anestesico; Allergan, Brazil) were applied to all animals. To avoid transmission of infectious ocular diseases, the prism from the Perkins tonometer was removed and washed in physiological saline solution after each use. It was then submerged for 10 min in a solution of 3% hydrogen peroxide, washed again in a physiological saline solution, and then dried with sterile gauze [13].
After measuring the IOP, the needle was removed from the anterior chamber. Soon after, cyanoacrylate glue was applied with a 25 × 7 hypodermic needle (Injex, Brazil) at the corneal puncture site to protect against fluid leakage [22]. After the procedure, the animals were given one drop of chloramphenicol eye drops (Visalmin; Bunker, Brazil), and diclofenac sodium eye drops, (Still; Allergan, Brazil) twice daily for 1 week. All animals underwent daily basic ophthalmic exams until the corneal lesions had healed.
The mean IOP values from ocular manometry and tonometry in the postmortem study were used to create a calibration curve. Perkins tonometer readings taken during the in vivo study were converted to IOP in mmHg using this calibration curve, and were analyzed by Student's t-tests. Linear regression analysis was performed to analyze the relationship between the postmortem manometry and tonometry IOP measurements, and the correlation coefficient (r2) was calculated. A p-value < 0.05 was considered to indicate statistical significance.
The correlation coefficients (r2) between the data from manometry and the Perkins tonometer were 0.866 in horses and 0.864 in cattle. For the postmortem study, the corresponding linear regression was y = 0.082x + 0.268 for horses, and y = 0.082 + 0.290 for cattle (Fig. 4). In the in vivo study, the IOP values measured by the manometer were 25.1 ± 2.9 mmHg (range, 19.0~30.0 mmHg) in horses (Table 1) and 19.7 ± 1.2 mmHg (range, 18.0~22.0) in cattle (Table 2). In the in vivo study, the mean IOP measured by the Perkins tonometer was 2.2 ± 0.3 (range 1.8~2.6 mmHg) in horses. This value corresponded to 23.4 ± 3.2 mmHg (range, 18.6~28.4 mmHg) according to our calibration curve (Table 1). In cattle (Table 2), the mean IOP measured by the Perkins tonometer was 1.8 ± 0.1 (range 1.6~2.0 mmHg) and corresponded to 18.8 ± 1.7 mmHg (range 15.9~20.8 mmHg) according to our calibration curve.
A recent study of using the Perkins tonometer on dogs and cats [1] spurred our interest in applying this tonometer to other species. Aside from the reduced cost of the Perkins tonometer, the advantages of this procedure observed in that study were increased accuracy, easy maintenance of the tonometer, and the ability to calibrate and disinfect the prism. A number of disadvantages were also associated with the Perkins tonometer including its narrow reading range (with an upper limit of 50 mmHg) and the longer training time necessary for its correct use. In the present study, identical advantages and disadvantages were observed with the use of the Perkins tonometer in horses and cattle.
There was a strong correlation between the IOP values obtained by direct ocular manometry and the Perkins tonometer in horses and cattle. The correlation coefficients for the Perkins tonometer in horses (r2 = 0.866) and cattle (r2 = 0.864) were very good. IOP readings taken by the Perkins tonometer are multiplied by 10 in humans [19], and a recently study demonstrated that the same factor should be used for dogs and cats [1]. Based on our calibration curves for horses (y = 0.082x + 0.268) and cattle (y = 0.082x + 0.290), we approximated the equation and multiplied Perkins tonometer reading by a factor of 10 for both horses and cattle. We therefore recommend calculating the mean of three readings and multiply this by a factor 10 when using the Perkins tonometer.
In our in vivo study, the mean IOP values calculated with the calibration curve from the Perkins tonometer data for horses (23.4 ± 3.2 mmHg; range, 18.6~28.4 mmHg) were very similar to the IOP directly measured by manometry (25.1 ± 2.9 mmHg; range, 19.0~30.0). The IOP values calculated from the Perkins tonometer data were also similar to those measured by the rebound (22.1 ± 5.9 mmHg; range, 10~34 mmHg) [11], Tonopen (23.3 ± 6.89 mmHg), and Mackay-Marg (23.5 ± 6.1 mmHg) tonometers [3,15]. The mean IOP values we calculated for cattle from the Perkins tonometer data and calibration curve (18.8 ± 1.7 mmHg; range, 15.9~20.8 mmHg) were also very similar to those directly measured by manometry (19.7 ± 1.2 mmHg; range, 18.0~22.0 mmHg). The calculated Perkins tonometry IOP values from our study were also close to those reported by Gerometta et al. [6] that were measured using the same tonometer (right eye: 16.1 ± 1.0 mmHg; range, 14.3~17.2 mmHg, and left eye: 16.5 ± 1.2 mmHg; range, 14.3~18.2 mmHg). However, the IOP values from our study were lower than those reported by Gum et al. [7], in Holstein and Jersey cows, using the Mackay-Marg tonometer were 27.5 ± 4.8 mmHg (range 16~39 mmHg), Mackay-Marg tonometer were 28.2 ± 4.6 mmHg (range 19~39 mmHg) and Tonopen XL tonometer were 26.9 ± 6.7 mmHg (range 16~42 mmHg).
We chose to use an aneroid manometer instead of an electronic monitor or mercury column manometer used in other reports [1,15,17] because the aneroid manometer has been shown to be an accurate, practical and low-cost option for taking experimental IOP measurements. The aneroid manometer is also easier to position at the correct height in relation to the eye. We did not compare the Perkins tonometer with other tonometers in horses and cattle in this study. Therefore, more research is needed to compare the accuracy of the Perkins tonometer with that of other tonometers [11].
Compared to other tonometers, the Perkins tonometer requires a longer training time to be used correctly in a clinical setting. Nevertheless, this tonometer has the distinct advantages of relatively high accuracy and low cost. The results of our experiment demonstrated that the Perkins handheld tonometer could be an additional method for accurately measuring IOP in equine and bovine eyes in the future.
Figures and Tables
Fig. 1
Postmortem study in an eye remaining within the orbits in the head of an adult horse. (A) A 23-gauge scalp vein needle connected via a polyethylene tube to a three-way stopcock The stopcock was also connected to a 10 mL reservoir of a 0.9% physiological saline solution with the stopcock in an open mode and to an aneroid manometer. (B) Cannulation of the anterior chamber using a 23-gauge scalp vein needle near the limbus. Cyanoacrylate glue was applied around the needle to prevent leakage of the aqueous humor.

Fig. 2
In vivo study in horses. (A) Auriculopalpebral nerve block anesthesia with 10 mL of lidocaine 2%. (B) Topical anesthesia with two eye drops of 1% tetracaine. (C) Fluorescein eye drop. (D) Perkins tonometer IOP reading. (E) Cannulation of the anterior chamber. (F) Aneroid manometer IOP reading of 20 mmHg.
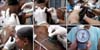
Fig. 3
In vivo study in cattle. (A) Auriculopalpebral nerve block localization. (B) Local anesthesia with 10 mL of 2% lidocaine. (C) Topical anesthesia with two eye drops of 1% tetracaine. (D) Fluorescein eye drop. (E) Perkins tonometer IOP reading. (F) Cannulation using a 23-gauge scalp vein needle to determine the IOP by aneroid manometry. (G) Application of cyanoacrylate glue. (H) Cyanoacrylate glue covering the site of cannulation. (I) Diclofenac sodium eye drop.

Fig. 4
Regression lines of IOP values from the Perkins applanation tonometer vs. ones from direct postmortem ocular manometry for the eyes of (A) horses (n = 10) and (B) cattle (n = 10).

References
1. Andrade SF, Cremonezi T, Zachi CAM, Lonchiati CF, Amatuzzi JD, Sakamoto KP, Mello PAA. Evaluation of the Perkins® handheld applanation tonometer in the measurement of intraocular pressure in dogs and cats. Vet Ophthalmol. 2009. 12:277–284.

2. Brooks DE, Ryan J. Gelatt KN, editor. Equine ophthalmology. Veterinary Ophthalmology. 1999. 3rd ed. Philadelphia: Lippincott Williams & Wilkins;1097–1115.
3. Dziezyc J, Millichamp NJ, Smith WB. Comparison of applanation tonometers in dogs and horses. J Am Vet Med Assoc. 1992. 201:430–433.
4. Frenkel REP, Hong YJ, Shin DH. Comparison of the Tono-Pen to the Goldmann applanation tonometer. Arch Ophthalmol. 1988. 106:750–753.

5. Gelatt KN, Peiffer RL Jr, Gum GG, Gwin RM, Erickson JL. Evaluation of applanation tonometers for the dog eye. Invest Ophthalmol Vis Sci. 1977. 16:963–968.
6. Gerometta R, Podos SM, Candia OA, Wu B, Malgor LA, Mittag T, Danias J. Steroid-induced ocular hypertension in normal cattle. Arch Ophthalmol. 2004. 122:1492–1497.

7. Gum GG, Gelatt KN, Miller DN, Mackay EO. Intraocular pressure in normal dairy cattle. Vet Ophthalmol. 1998. 1:159–161.

8. Hammond BR, Bhattacherjee P. Calibration of the Alcon applanation pneumatonograph and Perkins tonometer for use in rabbits and cats. Curr Eye Res. 1984. 3:1155–1158.

9. Kalesnykas G, Uusitalo H. Comparison of simultaneous readings of intraocular pressure in rabbits using Perkins handheld, Tono-Pen XL, and TonoVet tonometers. Graefes Arch Clin Exp Ophthalmol. 2007. 245:761–762.

10. Knollinger AM, La Croix NC, Barrett PM, Miller PE. Evaluation of a rebound tonometer for measuring intraocular pressure in dogs and horses. J Am Vet Med Assoc. 2005. 227:244–248.

11. Leiva M, Naranjo C, Peña MT. Comparison of the rebound tonometer (ICare®) to the applanation tonometer (Tonopen XL®) in normotensive dogs. Vet Ophthalmol. 2006. 9:17–21.

12. Lim KS, Wickremasinghe SS, Cordeiro MF, Bunce C, Khaw PT. Accuracy of intraocular pressure measurements in New Zealand white rabbits. Invest Ophthalmol Vis Sci. 2005. 46:2419–2423.

13. Lingel NJ, Coffey B. Effects of disinfecting solutions recommended by the Centers for Disease Control on Goldmann tonometer biprisms. J Am Optom Assoc. 1992. 63:43–48.
14. Maggs DJ. Maggs DJ, Miller PE, Ofri R, editors. Basic diagnostic techniques. Slatter's Fundamentals of Veterinary Ophthalmology. 2007. 4th ed. St. Louis: Saunders;81–106.

15. Miller PE, Pickett JP, Majors LJ. Evaluation of two applanation tonometers in horses. Am J Vet Res. 1990. 51:935–937.
16. Miller PE. Maggs DJ, Miller PE, Ofri R, editors. The glaucomas. Slatter's Fundamentals of Veterinary Ophthalmology. 2007. 4th ed. St. Louis: Saunders;230–257.

17. Passaglia CL, Guo X, Chen J, Troy JB. Tono-Pen XL® calibration curves for cats, cows and sheep. Vet Ophthalmol. 2004. 7:261–264.

18. Pickett JP, Ryan J. Equine glaucoma: a retrospective study of 11 cases from 1988 to 1993. Vet Med. 1993. 88:756–763.
19. Schottenstein EM. Ritch R, Shields MB, Krupin T, editors. Intraocular pressure and tonometry. The Glaucomas: Basic Sciences. 1996. 2nd ed. St. Louis: Mosby;407–428.
20. Takatsuji K, Sato Y, Izuka S, Nakatani H, Nakamura A. Animal model of closed angle glaucoma in albino mutant quails. Invest Ophthalmol Vis Sci. 1986. 27:396–400.
21. Van Der Woerdt A, Gilger BC, Wilkie DA, Strauch SM. Effect of auriculopalpebral nerve block and intravenous administration of xylazine on intraocular pressure and corneal thickness in horses. Am J Vet Res. 1995. 56:155–158.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download