Abstract
Epidemiological characteristics of swine pulmonary Pneumocystis (P.) carinii and concurrent infections were surveyed on Jeju Island, Korea, within a designated period in 172 pigs submitted from 54 farms to the Department of Veterinary Medicine, Jeju National University. The submitted cases were evaluated by histopathology, immunohistochemistry, PCR/RT-PCR, and bacteriology. P. carinii infection was confirmed in 39 (22.7%) of the 172 pigs. Histopathologically, the lungs had moderate to severe lymphohistioctyic interstitial pneumonia with variable numbers of fungal organisms within lesions. Furthermore, porcine reproductive and respiratory syndrome virus (PRRSV) and porcine circovirus type 2 (PCV-2) co-infection was a common phenomenon (12.8%, 20.5%, and 48.7% were positive for PRRS, PCV-2, or both, respectively, as determined by PCR/RT-PCR). Infection was much more concentrated during winter (December to March) and 53.8% of the infected pigs were 7- to 8-weeks old. In addition, three pigs showed co-infection with bacteria such as Pasteurella multocida and Streptococcus suis. The results of the present study suggest that the secondary P. carinii infection is common following primary viral infection in swine in Korea. They further suggest that co-infection of P. carinii might be enhanced by the virulence of primary pathogens or might have synergistic effects in the pigs with chronic wasting diseases.
Pneumocystis (P.) carinii is an opportunistic fungal pathogen of many animals as well as humans, and can cause fatal pneumonia in immune-compromised patients [13,20]. Pulmonary pneumocystosis has been reported in a wide variety of animals including rabbits, dogs, cats, horses, goats, pigs, chimpanzees, owl monkeys, and laboratory animals such as rabbits, mice, and rats [22]. In addition to the proper classification of Pneumocystis at the kingdom level, the additional DNA-sequence polymorphism of ribosomal RNA has shown that Pneumocystis organisms are quite diverse among different mammals [20]. The organism that causes human Pneumocystis pneumonia (PcP) is now named P. jiroveci.
Recently, chronic wasting diseases associated with porcine reproductive and respiratory syndrome virus (PRRSV) and postweaning multisystemic wasting syndrome (PMWS) have caused serious economic problems for domestic pig herds in many countries. Porcine circovirus type 2 (PCV-2) is nowadays considered the causal agent of PMWS [17]. Although PCV-2 might act as a primary pathogen in pigs, co-infection with other agents such as PRRSV, porcine parvovirus, pneumocystis, and Chlamydia spp. have been reported [2,5,17].
In Korea, P. carinii infections have been reported in humans with acquired immune deficiency syndrome (AIDS) or immunocompromised patients [16]. Recently P. carinii infection in two grower pigs reared in Jeju Island was reported [7]. Although this was regarded as an opportunistic pathogen, the epidemiologic status and the role of other respiratory agents should be clarified in Jeju Island, the warmest southern part of Korea.
This study reports on the results of a 2-year survey of swine P. carinii infection and concurrent respiratory diseases in Jeju Island, Korea, which found a 22.7% incidence of P. carinii infection.
A total of 172 pigs ranging from 4- to 15-weeks old, submitted to the Department of Veterinary Pathology, College of Veterinary Medicine, Jeju National University for diagnosis from 2006 to 2007, were included in this study. The pigs were from 54 private farms throughout Jeju Island, Korea.
After necropsy, all major parenchymal organs were fixed in 10% phosphate-buffered formalin, routinely processed, embedded in paraffin, and stained with hematoxlyin and eosin for light microscopy examination. During necropsy, portions of the lungs and tracheal or thoracic swabs were also collected aseptically for microbiological analysis. Replicate sections of the lungs with pneumonia were used for immunohistochemical identification of P. carinii with a commercially available mouse monoclonal antibody at a 1 : 50 dilution (Biodesign, USA) after 0.05% protease digestion. The standard streptavidin-biotin-peroxidase method with 3, 3-diaminobenzidine as the chromogen was used to identify the antigen according to the manufacturer's protocol (LSAB2 kit; Dako, USA). The lung sections were also stained using the periodic acid-Schiff (PAS) method to detect fungal elements.
Frozen lung tissues were analyzed by RT-PCR for the presence of PRRSV and by PCR for the presence of PCV-2. The tissues were homogenized with DNase- and RNase-free distilled water (Invitrogen, USA), and supernatants were stored at -70℃ until use. RNA and DNA were extracted from 200 µL of supernatants using an RNeasy Protect mini-kit (Qiagen, Germany) and a G-spin DNA extraction kit (iNtRON Biotechnology, Korea), respectively. All analyses were carried out using a Dice TP600 PCR thermal cycler (TaKaRa, Japan). For the RT-PCR, 2 µL of the RNA samples were added to 18 µL of a one-step RT-PCR mixture (Maxime RT-PCR premix; iNtRON Biotechnology, Korea). RT-PCR was carried out as previously described by Christopher-Hennings et al. [3] with minor modifications. After the one-step RT-PCR, nested PCR was performed. For the nested PCR, 2 µL of the DNA samples were added to 18 µL of PCR reaction mixture (Maxime PCR premix; iNtRON Biotechnology, Korea). PCR for PCV-2 was carried out as described by Larochelle et al. [12]. The amplified products were visualized by electrophoresis on a 1.2% agarose gel containing ethidium bromide.
Based on the animals' history, 140 pigs showed clinical signs of respiratory infection such as sneezing, cough, and dyspnea. At necropsy, 100 pigs had been runted and had rough coats along with moderate to severe lymphadenopathy, including mesenteric, inguinal, and submandibular lymph nodes. The lungs of 130 pigs failed to collapse and were rubbery in consistency, indicating interstitial pneumonia. Among these cases, other complicated lesions such as cranio-ventral consolidation (50 cases), fibrinous lobar pneumonia (three cases), and fibrino-purulent pleuritis (31 cases) were also observed. Bronchopneumonia (in four cases) and lobar pneumonia (in one case) were observed in pigs without viral lesions.
Other 37 pigs did not show any evidence of pneumonia. However, these pigs had several other types of lesions including enteritis (nine cases), edema disease (two cases), rectal stricture (two cases), dermatitis (one case), hernia (one case), and other minor lesions.
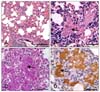
Among the 172 pigs examined histologically, 39 (22.7%) showed pulmonary lesions compatible with P. carinii infection. There was moderate to severe thickening of the alveolar septa due to infiltration of alveolar macrophages and lymphocytes (Fig. 1A). Alveoli were often filled with macrophages, lymphocytes, fibrin, and various numbers of round to ovoid yeast-like structures (Fig. 1B). The yeast-form structures were positive for PAS staining (Fig. 1C). The organisms were 3 to 5 µm in diameter, and were found freely in the alveolar lumen, cytoplasm of alveolar macrophages, and alveolar septa. Immunohistochemically, the organisms were strongly positive for P. carinii (Fig. 1D).
Based on the histopathologic classification of pneumonia, mild to severe lympho-histiocytic bronchointerstitial pneumonia with occasional secondary suppurative (bacterial) pneumonia characterized by alveolar thickening and peribronchiolar fibroplasia was noted in 139 pigs. Among these animals, IHC showed that 32 pigs were positive for P. carinii. Other pneumonia such as acute suppurative bronchopneumonia and fibrinous hemorrhagic lobar pneumonia were noted in 4 pigs and one pig, respectively. Among these five pigs, three cases were positive for P. carinii. Other 28 animals showed digestive system lesions such as colibacillosis, ulcerative colitis, edema disease, and intestinal spirocheatosis without any or with only subtle respiratory problems. Among these 28 pigs, four cases were positive for the antigens of P. carinii.
RT-PCR analyses yielded 236-bp products for PRRSV, and PCR analysis yielded 263-bp products for PCV-2. Among the 172 pigs, the overall positive rates of PRRSV, PCV-2, and both PRRSV and PCV-2 were 19.8% (34 cases), 15.1% (26 cases), and 45.9% (79 cases), respectively. Among these 139 virally-infected pigs, one or more bacterial pathogens were detected from 110 pigs (79.1%). The most commonly isolated bacteria were Streptococcus suis (25.2%) followed by Mycoplasma hyopneumoniae (20.1%), Pasteurella multocida (12.9%), Actinobacillus pleuropneumoniae (5.0%), and minor pathogens such as Salmonella spp. and Bordetella bronchiseptica. In addition, Pasteurella multocida (two cases), Streptococcus suis (two cases), and Actinobacillus pleuropneumoniae (one case) were isolated in five pigs (2.9%) in the absence of viral infection. The other 28 virally-infected pigs (16.3%) were not further infected with any respiratory pathogens. However, these 28 pigs suffered from digestive disorders or dermatologic problems.
Immunohistochemistry detected P. carinii in 22.7% (39/172) of the total lung samples analyzed (Table 1). Among 39 pigs with P. carinii infection, five pigs (12.8%), eight pigs (20.5%) and 19 pigs (48.7%) were positive for PRRSV, PCV-2, and dually positive for PRRSV and PCV-2, respectively. Three P. carinii-positive pigs (7.7%) also had bacterial infection. The remaining four P. carinii-positive pigs were free of respiratory pathogens. In summary, P. carinii infection was detected in 23.0% (32/139) of the virus-infected pigs and in 14.3% (4/28) of the pigs with no viral pathogens detected. Among the 39 pigs infected with P. carinii, 32 pigs (82.0%) were co-infected with virus, three pigs (7.7%) were co-infected with bacteria, and four pigs (10.3%) were not co-infected with any respiratory pathogens.
According to geographic distribution, 20 P. carinii-positive pigs were from Jeju City in the northern part of Jeju Island, and 19 pigs were from Seogwipo City in the southern part of the island. P. carinii were detected immunohistochemically in the pigs from 4 to 14 weeks of age. However, the majority of cases was observed among 7-week-old (12 cases) and 8-week-old (9 cases) animals (Fig. 2). And this disease occurred throughout the year (Fig. 3). However, the prevalence was higher in the cold season (December to March; 23 cases) than in the hot season (June to September; 10 cases).
Based on the immunohistochemistry and special staining and histopathology, 22.7% of the pigs on Jeju Island with pneumonia were also infected with P. carinii. About half of the infected pigs (53.8%) were from 7 to 8 weeks of age. The highest number of cases occurred in the winter cold season. In addition, PRRSV and PCV-2 were the primary co-infecting pathogens in most of positive cases.
PcP was first described in young piglets with exudative pneumonia in 1958 [1]. Since then, spontaneous porcine PcP has been reported only rarely in some countries [6,7,11,18]. Kucera et al. [11] demonstrated P. carinii pneumopathy in three experimental pigs suffering from hypervitaminosis-D. A previous study described an outbreak of epidemic porcine pneumonia on a "farrow-to-finish" pig farm in Japan due to P. carinii that resembled the infantile epidemic form of PcP in humans [10].
The predisposing factors responsible for the outbreak of porcine PcP are not clear. Settnes and Henriksen [18] thought that the stress of weaning might predispose pigs to PcP. One survey indicated that the pneumonia associated with P. carinii occurs in conjunction with the piglets' exposure to stress factors such as early weaning, new environments, new pen mates, overcrowding, and a change of diet as well as exposure to other diseases [1]. Although no obvious gross lesions were noticed in lung samples, P. carinii was found to be more prevalent in growth-retarded pigs than in healthy pigs [6]. This suggests that unexplained defective immune functions in the growth-retarded pigs might be associated with porcine PcP. Kondo et al. [10] examined epidemic porcine PcP in a pig farm. The clinical disease was observed in weanling piglets, and P. carinii organism and pneumonia were found in 18 (78.3%) of the 23 pigs examined. Most lesions associated with P. carinii were detected in the pigs at 5~10 weeks of age, the stage of physiological hypogammaglobulinaemia.
In the present survey, P. carinii antigens were detected immunohistochemically in the pigs at 4~14 weeks of age. Similar to that study, most antigen-positive pigs were concentrated among 7- to 8-week-olds. Nielsen et al. [15] demonstrated that the prolonged treatment with a high dose of methylprednisolone acetate causes involution of the thymus and lymphopenia concurrently with the reactivation of latent P. carinii in seropositive piglets. This group suggested that immunodeficiency was closely related to the development of PcP in pigs.
Both the cellular and humoral immune systems are important defenses against Pneumocystis infection. CD4+ T lymphocytes are critical as most human patients with PcP have a very low CD4+ T-cell count [13]. Pneumonia due to P. carinii is the most common AIDS-defining disease in which a decrease in the number of circulating CD4+ T cells leads to the development of pneumonia. A decrease in the number of CD4+ T cells has also been described in the early stage of PRRSV infection [19]. Lymphocyte depletion of follicular and interfollicular areas, together with macrophage infiltration of lymphoid tissues, contributes to the development of the unique lesion that is the basic feature of PMWS-affected pigs. These findings are highly correlated with the decrease of circulating B- and T-cells in PCV-2-infected pigs [17]. Therefore, PRRSV and PCV-2 are capable of suppressing immune functions to a degree sufficient for making the pigs more susceptible to secondary infections [17]. It is thought that a PRRSV infection might exacerbate P. carinii infection resulting in the pneumonia observed in the pigs from a herd with epidemic pneumonia [9]. This study demonstrated that the incidence of P. carinii in virally-infected pigs (23.0%) was higher than in the pigs (14.3%) without additional viral or bacterial infection. Therefore, it is reasonable to speculate that PRRSV- and/or PCV-2-induced immunosuppression enhances susceptibility to PcP in pigs.
According to a previous study, there are no regional or seasonal variations in the level of P. carinii infection in pigs [8]. However, it is well-known that the incidence of many viral and bacterial respiratory tract infections is subject to seasonal fluctuations. Some studies in European countries showed the PcP incidence in people with or without HIV infection to be maximal in the winter months, especially from January to March, similar to other infectious diseases [14,21]. In this study, 59% of P. carinii infections in pigs occurred from December to March. This result suggests that airborne transmission of P. carinii is more prevalent in the closed living condition during winter than in the open living condition during warmer seasons.
The detection rate of P. carinii in pigs that were also infected with PCV-2 ranged from 2% to 5% in Canada [4,5] to 28% among slaughtered pigs in Brazil [2]. In the present study, we detected a 25.7% (27/105) prevalence rate for P. carinii in pigs that were co-infected with PCV-2. This was significantly higher than the 2% or 5% prevalence for this fungus in PCV-2 infected cases previously reported in Canada.
The results of this study showed that PcP is prevalent in diseased pigs on Jeju Island, especially in virally-infected cases. The highest number of P. carinii cases occurred in the winter. To control the dissemination of this disease and minimize the economic loss to the pig industry, effective preventive and therapeutic measures are warranted.
Figures and Tables
Fig. 1
Lung of pig. (A) Diffuse lymphohistiocytic interstitial pneumonia. (B) Note the honeycomb materials (arrow) in the alveolar lumens. (C) and (D) Note the numerous round cysts (arrows) about 4-6 µm in diameter along the alveolar wall and within the alveolar lumen. A and B: H&E stain. C: PAS stain. D: Streptavidin-biotin peroxidase stain. Scale bars = 200 µm (A), 50 µm (B-D).
Acknowledgments
This study was supported by a grant (code# 20070401034 009) from the BioGreen 21 Program, Rural Development Administration, Korea. We also thank all the practitioners who submitted the cases for this study.
References
1. Bille-Hansen V, Jorsal SE, Henriksen SA, Settnes OP. Pneumocystis carinii pneumonia in Danish piglets. Vet Rec. 1990. 127:407–408.
2. Cavallini Sanches EM, Borba MR, Spanamberg A, Pescador C, Corbellini LG, Ravazzolo AP, Driemeier D, Ferreiro L. Co-infection of Pneumocystis carinii f. sp. suis and porcine circovirus-2 (PCV2) in pig lungs obtained from slaughterhouses in southern and midwestern regions of Brazil. J Eukaryot Microbiol. 2006. 53:Suppl 1. S92–S94.
3. Christopher-Hennings J, Nelson EA, Nelson JK, Hines RJ, Swenson SL, Hill HT, Zimmerman JJ, Katz JB, Yaeger MJ, Chase CCL, Benfield DA. Detection of porcine reproductive and respiratory syndrome virus in boar semen by PCR. J Clin Microbiol. 1995. 33:1730–1734.

4. Clark EG. Post-weaning multisystemic wasting syndrome. Proc Am Assoc Swine Pract. 1997. 28:499–501.
5. Ellis J, Hassard L, Clark E, Harding J, Allan G, Willson P, Strokappe J, Martin K, McNeilly F, Meehan B, Todd D, Haines D. Isolation of circovirus from lesions of pigs with postweaning multisystemic wasting syndrome. Can Vet J. 1998. 39:44–51.
6. Fujita M, Furuta T, Nakajima T, Kurita F, Kaneuchi C, Ueda K, Ogata M. Prevalence of Pneumocystis carinii in slaughtered pigs. Nippon Juigaku Zasshi. 1989. 51:200–202.
7. Jung JY, Kim KS, Kim DY, Kim JH. Pneumocystis carinii pneumonia in pigs. Korean J Vet Res. 2007. 47:321–324.
8. Kondo H, Hikita M, Ito M, Kadota K. Immunohistochemical study of Pneumocystis carinii infection in pigs: evaluation of Pneumocystis pneumonia and a retrospective investigation. Vet Rec. 2000. 147:544–549.

9. Kondo H, Kuramochi T, Taguchi M, Ito M. Serological studies on porcine Pneumocystis carinii pneumonia: kinetics of the antibody titers in swine herds and the association of porcine reproductive and respiratory syndrome virus infection. J Vet Med Sci. 1997. 59:1161–1163.

10. Kondo H, Taguchi M, Abe N, Nogami Y, Yoshioka H, Ito M. Pathological changes in epidemic porcine Pneumocystis carinii pneumonia. J Comp Pathol. 1993. 108:261–268.

11. Kucera K, Slesinger L, Kadlec A. Pneumocystosis in pigs. Folia Parasitol (Praha). 1968. 15:75–78.
12. Larochelle R, Antaya M, Morin M, Magar R. Typing of porcine circovirus in clinical specimens by multiplex PCR. J Virol Methods. 1999. 80:69–75.

14. Lubis N, Baylis D, Short A, Stebbing J, Teague A, Portsmouth S, Bower M, Nelson M, Gazzard B. Prospective cohort study showing changes in the monthly incidence of Pneumocystis carinii pneumonia. Postgrad Med J. 2003. 79:164–166.

15. Nielsen J, Bille-Hansen V, Settnes OP. Experimental corticosteroid induction of Pneumocystis carinii pneumonia in piglets. APMIS. 1999. 107:921–928.

16. Park SY, Lee HK. Combined Pneumocystis carinii pneumonia and miliary tuberculosis in a patient with AIDS. Korean J Pathol. 1994. 28:657–662.
17. Segalés J, Domingo M, Chianini F, Majó N, Domínguez J, Darwich L, Mateu E. Immunosuppression in postweaning multisystemic wasting syndrome affected pigs. Vet Microbiol. 2004. 98:151–158.

18. Settnes OP, Henriksen SA. Pneumocystis carinii in large domestic animals in Denmark. A preliminary report. Acta Vet Scand. 1989. 30:437–440.

19. Shimizu M, Yamada S, Kawashima K, Ohashi S, Shimizu S, Ogawa T. Changes of lymphocyte subpopulations in pigs infected with porcine reproductive and respiratory syndrome (PRRS) virus. Vet Immunol Immunopathol. 1996. 50:19–27.

20. Stringer JR, Beard CB, Miller RF, Wakefield AE. A new name (Pneumocystis jiroveci) for Pneumocystis from humans. Emerg Infect Dis. 2002. 8:891–896.
21. Varela JM, Regordán C, Medrano FJ, Respaldiza N, de la Horra C, Montes-Cano MA, Calderón EJ. Climatic factors and Pneumocystis jiroveci infection in southern Spain. Clin Microbiol Infect. 2004. 10:770–772.
22. Wazir JF, Ansari NA. Pneumocystis carinii infection. Update and review. Arch Pathol Lab Med. 2004. 128:1023–1027.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download