Introduction
Atherosclerotic vascular disease and the resulting sequelae remain the leading cause of death in developed countries worldwide. Stenting is the most frequently performed percutaneous intervention for the treatment of coronary artery disease. However, re-occlusion due to stent thrombosis in the early period and in-stent restenosis in the later period after stent deployment are principal drawbacks of coronary stenting. To attenuate in-stent restenosis, many researchers have studied the effects of new polymers, metals, stent architecture, and drugs on the inhibition of neointimal hyperplasia.
The animal models most commonly used to assess vascular responses to stent implantation are the porcine coronary artery model [19,27] and the rabbit iliac artery model [2,13]. The porcine coronary artery stenting model [11,24] is suitable for research because the porcine heart is similar to the human heart in terms of pathologic and physiologic vascular responses. However, the rapid growing pigs results in a high cost of breeding. The rabbit iliac artery stenting model [7,13] has the advantage of a low cost but the pathologic and physiologic responses of rabbits differ from those in humans. In addition, it is widely recognized that arterial repair after stent placement in animals occurs more rapidly than in humans although the sequence of biological events associated with healing are remarkably similar [28]. However, Finn et al. [9] reported a significant difference in the rate of healing between the porcine model and the rabbit model. After placement of a bare metal stent (BMS), the porcine model showed completed endothelialization by 14 days whereas it took 21 days to complete this process in the rabbit model.
Previous studies in the porcine or rabbit model used both drug-eluting stents and BMS [2,4]. In this study, to demonstrate the validity of our novel rat model in comparison with these previous animal models, we implanted BMS and paclitaxel-eluting stents (PES) and assessed stent re-endothelialization, neointimal hyperplasia, and thrombus formation 2 and 4 weeks after stent implantation.
Materials and Methods
Animal care, stent deployment, and aortogram
All animal protocols were approved by the Chonnam National University Animal Care and Use Committee (CNU IACUC-H-2009-18). Male Sprague-Dawley rats were purchased from Samtako (Korea). All rats (b.w. 400 ± 20 g) were fed a normal pelleted diet and were given water ad libitum. Starting 1 week before stent implantation and continuing throughout the study period, the rats' drinking water contained aspirin (3.25 mg/kg; Bayer Vital, Germany) and clopidogrel (0.75 mg/kg, Plavix; Sanofi-Aventis, USA).
In the baseline study, a total of 14 rats underwent an aortogram and surgery. We measured the aorta size of 10 rats by angiogram before stent implantation. To select the optimal stent size (diameter and length), balloon pressure, and stent-to-artery ratio, the aorta size of the rats that underwent stent implantation was measured by angiogram. A balloon pressure of 9 atm resulted in a stent-to-artery ratio into 1:1.2. A BMS (n = 6, Liberté 3.5 × 20 mm; Boston Scientific, USA) or a PES (n = 6, Taxus Liberté 3.5 × 20 mm; Boston Scientific, USA) was deployed at 9 atm in the rats.
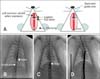
For stent implantation, the rats were anesthetized with an intraperitoneal injection of ketamine (50 mg/kg; Bayer Animal Health, Korea) and xylazine (6.7 mg/kg; Bayer Animal Health, Korea), and the left common carotid artery was surgically exposed. The proximal portion of the left common carotid artery was ligated with 5-0 silk thread. The distal portion was then occluded by a vessel clamp to block blood flow and a carotid cut down was performed at the mid portion. Under fluoroscopic guidance [7,18], the stent catheter was advanced to the thoracic aorta via the left common carotid arteriotomy. A 0.36 mm percutaneous transluminal coronary angioplasty (PTCA) guide wire was advanced into the thoracic aorta. A BMS or PES with a balloon-to-artery ratio ranging from 1:1.2 to 1:1.3 was placed at the desired location in the thoracic aorta and inflated to 9 atm of balloon pressure. After stent deployment, the animals were allowed to recover in their normal housing at the animal care facility. The animals were monitored daily until the subsequent experimental procedures and euthanasia 2 or 4 weeks after stent placement.
Aortography was performed 2 or 4 weeks after stent deployment (Fig. 1A) using a contrast agent (Visipaque; Amersham Health, Ireland). An angiography catheter (18 G radiology needles; BD, USA) was introduced through the right common carotid artery and advanced to the thoracic aorta to deliver the contrast agent. Aorta size was calculated by quantitative angiography before stent implantation. Stent size and balloon pressure were also determined. The stent-to-artery ratio was calculated by dividing the post-implant aorta diameter by the pre-implant aorta diameter.
Tissue preparation and histomorphometric analysis
At 2 and 4 weeks after stent implantation, the rats were anesthetized with an intraperitoneal injection of ketamine and xylazine. After the final aortogram, the aorta was flushed gently with a 0.9% saline solution and perfusion fixation was performed with 2.5% glutaraldehyde. The stented aorta was harvested with at least 1 cm of the proximal and distal margin. After an additional 24 h of immersion fixation with 2.5% glutaraldehyde, the stented aorta was cut into two segments.
One segment of the stented aorta was used for scanning electron microscopy (SEM) analysis. SEM analysis was conducted by following standard procedures. In brief, the stented aorta was fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (Sigma Aldrich, USA) for 2 h and then OsO4 for 90 min, followed by dehydration through a series of alcohols. The samples then underwent critical point drying before being coated with evaporated gold and observed under an S-3500N scanning electron microscope (Hitachi, Japan). The re-endothelialization ratio was calculated by using NIS-Elements (version 3.00 SP7; Nikon, Japan).
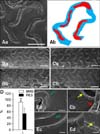
The other segment of the stented aorta was placed in 10% formalin for an additional 24 h. Cross-sections were prepared from the segment by embedding the tissue in acrylic plastic and cutting sections 75 µm-thick. All sections were stained with hematoxylin and eosin. Histomorphometric analysis was carried out using Nis-Elements. The neointimal area was calculated as the media area minus the inner luminal area (neointimal area = media area - inner luminal area). These areas were determined in each section, averaged, and expressed as the absolute area in mm2. Additionally, the uncovered strut ratio was determined by microscopic observation and calculated as exposed strut number / total strut number. The focal thrombus ratio was calculated as thrombus-containing strut number / total strut number as found by microscopic observation. These were averaged over all stained sections. At SEM analysis, the re-endothelization percentage (%) was calculated as the strut area minus the uncovered area divided by the strut area (re-endothelization percentage (%) = strut area - uncovered area / strut area) (Fig. 4Ab).
Statistical analysis
Measurements for each stented aorta (total sections from the mid to distal portion) were averaged to produce a mean value per stent. Tests for statistical significance were two-tailed, and significance was established by a p-value < 0.05 (v 16.0; SPSS, USA). Unless otherwise noted, data are represented as the mean ± SD.
Results
Establishment of the rat aorta stent model
The total procedure time for stent implantation in the rat aorta was approximately 15 to 20 min. Four rats died during surgery due to bleeding from the carotid artery at the site of incision. All stented aorta (n = 12) in the surviving rats were patent. The operation procedure is illustrated in Fig. 1A. The left common carotid artery was exposed and PTCA guide wire was inserted into the carotid artery. The stent was then inserted into the thoracic aorta via the guide wire (Figs. 1B and C). At 2 or 4 weeks after stent implantation, angiography was performed with an 18 G IV catheter in the right carotid artery (Fig. 1D). Quantitative angiograms at each time point (2 or 4 weeks after stenting) showed that the stents were implanted with similar stent-to-artery ratios.
Histomorphometric analysis
The neointimal area was larger in the BMS group (0.262 ± 0.010 mm2) than in the PES group (0.170 ± 0.014 mm2) at 2 weeks following stent placement (p < 0.05) (Figs. 2A and 3A). However, no significant difference in the neointimal area was observed between the two groups [BMS group: 0.567 ± 0.055 mm2 vs. PES group: 0.618 ± 0.089 mm2 (p > 0.05)] at 4 weeks (Figs. 2A and 3C). The uncovered strut ratio was 38.3 ± 16.5% in the BMS group and 84.2 ± 1.1% in the PES group at 2 weeks, and 8.3 ± 4.0% in the BMS group and 45.0 ± 7.1% in the PES group at 4 weeks (Fig. 2B). The focal thrombus ratio was 0.0 ± 0.0% in the BMS group and 29.4 ± 8.9% in the PES group at 2 weeks and 5.0 ± 7.1% in the BMS group and 51.3 ± 8.3% in the PES group at 4 weeks. In addition, thrombi were detected only in the neointimal area (Figs. 2C and 3D).
SEM evaluation of re-endothelialization
At SEM analysis, the portion of the stent struts that remained uncovered was less in the BMS group than in the PES group at 2 weeks after stent implantation. Stent detachment was observed in the PES group (Fig. 4B). As seen in Figs. 4C and D, the portion of the stent struts that was covered increased in both groups at 4 weeks. The BMS struts were mostly covered (93 ± 4%), but the PES struts were not sufficiently covered (53 ± 18%) (p < 0.05). The strut was covered by endothelial cells in the BMS group (Fig. 4E, green arrows). In the PES group, non-endothelial cells such as inflammatory cells and platelets were detected on the stent surface (Fig. 4E, yellow arrows).
Discussion
In this study, we developed a novel aorta stent model in rats that is similar to percutaneous implantation of a coronary stent in human, and compared the effects of BMS with those of PES. Our aorta stent model in rats has several advantages. First, the stent site was not exposed to the external environment. Therefore, it was protected from complications caused by inflammation and tissue adhesion. In addition, stent implantation was performed using human stent implantation devices. Rats are ideal for biochemical and biological analyses compared to pig models because they can be easily studied with various commercially available antibody and ELISA kits. In addition, rats have various disease models such as transgenic, obese, atherosclerotic, diabetic, and hypertensive strains Moreover, knockout rat strains are becoming increasingly available.
Langeveld et al. [18], Indolfi et al. [12], and Finn et al. [7] used an open abdominal aorta stent rat model or deployed a small-caliber stent into the carotid artery in the rat. The open abdominal aorta model is very useful, but exposure of the abdominal cavity and abdominal aorta to the external environment may cause reactions such as inflammation and adhesion. Additionally, the rat carotid artery cannot be accessed by commercially manufactured coronary stents. Our model did not require microsurgical equipment or special skills. Furthermore, we used commercially available BMS and PES in the rat thoracic aorta.
In previous report, PES has been shown to reduce the rate of restenosis and late lumen loss compared to BMS, resulting in a significant reduction in the need for target vessel revascularization [23]. However, an increased risk of late stent thrombosis (defined as thrombosis ≥ 30 days after stent deployment) has been identified as a major safety concern [1,3,6,14-17,21,22,25,26]. This concept is based on clinical autopsy and intracoronary angioscopic studies [5,10,16]. In the present study, the aorta stent model in SD rats showed vascular reaction patterns of re-endothelialization, neointimal induction, and thrombus formation similar to those in a swine model [8-10]. However, the induction of neointimal hyperplasia was weaker in our rat model than in the swine model.
By SEM analysis, the percentage of the stent area covered in the rats was found to be over 90% in the BMS group and about 50% in the PES group at 4 weeks after stent implantation. Experiments in a porcine coronary stent model have suggested that re-endothelialization was complete at 28 days after stent placement [8]. However, in another study, re-endothelialization was clearly delayed when a PES was implanted in a rabbit iliac artery [4].
The histological analysis showed that the neointimal area was increased at 4 weeks compared to 2 weeks. The extent of neointimal hyperplasia was less in the PES group than in the BMS group at 2 weeks. In the PES group, decreased neointimal hyperplasia was related to the paclitaxel coating of the stent strut surface. No difference in neointimal hyperplasia was observed 4 weeks after stenting between the BMS and PES group. This phenomenon might be called "late catch-up." In the BMS group, neointimal formation gradually increased between 2 and 4 weeks after stent implantation. In the PES group, however, reduced neointimal formation observed until 2 weeks suddenly increased between 2 and 4 weeks after stent implantation.
Thrombus formation could be a possible factor in late catch-up. In our study, thrombi were detected in the PES group in the outer area of the neointima at 2 weeks and in the inner area of the neointima at 4 weeks. From our experiments, we can hypothesize that the PES inhibited neointimal formation during the early phase, but caused thrombus formation and restenosis during the later phase.
In conclusion, we have demonstrated the creation of a novel model of aorta stenting via the common carotid artery in rats. Our model showed similar vascular responses after implantation of BMS and PES in normal rat aorta compared to pig and rabbit models. This model had the disadvantage of weaker neointimal hyerplasia compared to the pig coronary artery stent model [20], but had the advantages of enabling the detection of thrombi, re-endothelialization analysis, histomorphometric analysis, easy handling, and low cost. In addition, our model will allow relative long-term follow-up and research on aged animals because of the short life-span.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download