Abstract
Mammalian oocyte maturation and early embryo development processes are Ca2+-dependent. In this study, we used confocal microscopy to investigate the distribution pattern of Ca2+ and its dynamic changes in the processes of bovine oocytes maturation, in vitro fertilization (IVF), parthenogenetic activation (PA) and somatic cell nuclear transfer (SCNT) embryo development. During the germinal vesicle (GV) and GV breakdown stage, Ca2+ was distributed in the cortical ooplasm and throughout the oocytes from the MI to MII stage. In IVF embryos, Ca2+ was distributed in the cortical ooplasm before the formation of the pronucleus. In 4-8 cell embryos and morulas, Ca2+ was present throughout the blastomere. In PA embryos, Ca2+ was distributed throughout the blastomere at 48 h, similar to in the 4-cell and 8-cell phase and the morula. At 6 h after activation, there was almost no distribution of Ca2+ in the SCNT embryos. However, Ca2+ was distributed in the donor nucleus at 10 h and it was distributed throughout the blastomere in the 2-8 cell embryos. In this study, Ca2+ showed significant fluctuations with regularity of IVF and SCNT groups, but PA did not. Systematic investigation of the Ca2+ location and distribution changes during oocyte maturation and early embryo development processes should facilitate a better understanding of the mechanisms involved in oocyte maturation, reconstructed embryo activation and development, ultimately improving the reconstructed embryo development rate.
Heilbrunn found that Ca2+ had complex and multiple functions in biological systems and was the basic characteristic of all living cells [13]. It has also been suggested that Ca2+ was a widespread signaling molecule that played important roles in the transduction of Ca2+ signals to regulate various cellular processes [1], and that it was involved in almost all physiological processes in cells including secretion, contraction, proliferation, development, gene expression, and programmed cell death [2,20]. Furthermore, genetic and biochemical evidence support a role of Ca2+ in mitosis. In contrast, there has been a long-standing debate as to whether Ca2+ signals were required for oocyte meiosis [38]. In 1992, Carroll first reported that spontaneous Ca2+ fluctuations existed during in vitro maturation (IVM) of mouse oocytes, and that such fluctuations may be related to cytoplasm maturation [7]. Tombes found that IVM of mouse oocytes did not depend on changes in intracellular calcium [41], and Wang reached the same conclusion in an experiment conducted using mice [44]. However, Sun showed that cytoplasmic Ca2+ played a dual role during Xenopus oocyte maturation [39]. Homa found that intracellular free Ca2+ was a messenger of intrinsic signals that participated in regulating oocyte meiotic processes by microinjection of Ca2+ promoter and inhibitors [14]. Mehlmann also pointed out that Ca2+ chelator can delay the occurrence of meiosis [25]. In Xenopus, early reports argued that an increase in Ca2+ is sufficient to induce oocyte maturation, and that injection of Ca2+ buffers blocks such maturation [11].
Oocyte activation is made possible by fertilization with sperm, which results in an increase of intracellular free [Ca2+]i [22]. This increase in [Ca2+]i is necessary and the most effective signal for completion of all of the events involved in oocytes activation [15], which include the release of cortical granules (CG), exit from MII stage arrest, completion of meiosis, recruitment of the maternal mRNAs and achievement of the cell-cycle progression into interphase [34]. This [Ca2+]i signal is delivered in the form of long-lasting [Ca2+]i oscillations that begin shortly after fusion of the gametes and persist beyond the time of meiosis completion [7]. The release of zygote endogenous Ca2+ induced [Ca2+]i oscillation and formed the first cleavage signal to start early embryonic development [9]. A Ca2+ increase was recorded in sea urchin eggs at fertilization [35,46], and the calcium ionophore A23187 was shown to activate both vertebrate and invertebrate eggs and oocytes [36].
Although oocytes activation and embryo development in all species is accomplished using a wide range of [Ca2+]i patterns, a consensus is emerging that a physiological pattern of oscillations, such as those induced by sperm, result in greater embryo survival [47]. Consistent with this evidence, distinct events of oocytes artificial and fertilized activation were initiated and completed by different numbers of [Ca2+]i increases [23]. Moreover, effective activation of reconstructed embryos was found to be one of the important factors involved in nuclear transfer (NT) [40]. However, reports of Ca2+ dynamic changes and the distribution of Ca2+ in bovine oocytes and in vitro embryos were not observed. Therefore, in this study, laser scanning confocal microscope was used to investigate the distribution pattern of Ca2+ and its dynamic changes in different regions of cells in the processes of bovine oocytes maturation, in vitro fertilization (IVF), parthenogenetic activation (PA) and somatic cell nuclear transfer (SCNT) embryonic development to identify reasons for low development so that embryo development rates can be further improved.
All reagents used were obtained from Sigma (USA), unless otherwise stated. M199 was obtained from Gibco (USA), Pluronic F-127 and Fluo-3/AM were obtained from Invitrogen (USA) and fetal bovine serum (FBS) was purchased from Biochrom (Germany). Oocytes were collected from abattoir-derived ovaries in Dachang County, Hebei Province, China.
Oocytes with more than 3-layer packets of cumulus cells were cultured in fresh preheating M199 medium (Gibco, USA) and then incubated at 38.5℃ in a humidified atmosphere of 5% CO2 in air for about 20~24 h.
Denuded mature oocytes were incubated in a drop of cytochalasin B (CCB) with Hoechst 33342 (Sigma, USA) for 10 min, after which they were enucleated with a micro glass pipette by aspirating the first polar body and chromosomes in the MII phase with a small volume of surrounding cytoplasm. Following aspiration, an aliquot of the donor cell suspension was transferred to CCB supplemented with 10% polyvinylpyrrolidone (Sigma, USA). The donor cell was then gently injected into a separate enucleated oocyte.
Fertilization was conducted after oocytes was matured in vitro for 25 h. Briefly, semen was thawed at 38℃ for about 40 sec, after which it was slowly added to the bottom of a centrifuge tube containing Bracket and Oliphant's (BO) medium [29] and allowed to pre-equilibrate for 2 h. The tube was subsequently placed in a 38.5℃ incubator for more than 1 h to allow the sperm to swim out and capacitate. Next, the supernatant was transferred to another tube and centrifuged for 15 min at 1,500 rpm, after which the supernatant was discarded. The concentration of the sperm mixture was determined using a counting chamber and adjusted to 6 × 106 sperm/mL. Matured oocytes with 2~3 layers granulosa cells were placed into separate 50 µL microdrops containing BO medium-bovine serum albumin and then covered with mineral oil. The capacitated sperm were then added to the microdrops. Following co-incubation for 12~16 h, the oocytes were transferred to synthetic oviducal fluid (SOF) medium [33] and cleavage was observed. On day 2 of culture, the embryo cleavage rate was assessed and half of the medium was replaced with 10% FBS. On day 7, the blastocyst rate was assessed.
At 2 h after injection, oocytes or reconstructed embryos were washed three times with PBS, activated in 10 µmol/L ionomycin for 5 min and then in 2 mmol/L N-6 dimethylaminopurine (Sigma, USA) for 3.5~4 h. Finally, the activated oocytes or embryos were cultured in SOF medium at 38.5℃ in a humidified atmosphere of 5% CO2.
Oocytes or reconstructed embryos were washed three times with PBS, after which they were incubated in SOF medium with 6 µmol/L Fluo-3/AM for 30~40 min, washed with SOF 3~5 times to remove the Fluo-3/AM, and finally observed using a laser scanning confocal microscope (Nikon, Japan).
Investigation of the distribution and fluorescent intensity of Ca2+ in oocytes of the germinal vesicle (GV), GV breakdown (GVBD), MI, MII phase.
Investigation of the distribution and fluorescent intensity of Ca2+ in IVF embryos at different developmental stages.
Investigation of the distribution and fluorescent intensity of Ca2+ in parthenogenetic activated embryos at different developmental stages.
Investigation of the distribution and fluorescent intensity of Ca2+ in SCNT embryos at different developmental stages.
Fluorescent intensity was tested using the software freeviewer for laser scanning confocal microscopy and analyzed using SAS (version 8.13; SAS, USA). A p < 0.05 was considered to indicate significance for discrete and continuous analyses.
During the GV stage, Ca2+ was primarily distributed in the cortical ooplasm region and the fluorescence intensity was weak, while there was almost no distribution in other regions (Fig. 1A). In the GVBD stage (after 8 h of IVM), Ca2+ was still distributed in the cortical ooplasm region and tended to diffuse to the center of cells (Figs. 1B and C). From the MI to MII stage, Ca2+ was distributed throughout the oocytes. In the MI stage (after 14 h of IVM), the fluorescence intensity of the cortical ooplasm region was stronger than in the cytoplasmic area (Fig. 1D), while at the end of the MI stage Ca2+ in the chromosome area was higher (Fig. 1E), while there was stronger fluorescence in the polar bodies in the MII stage (Fig. 1F).
When the GVBD stage and MI stage were compared, the Ca2+ fluorescence intensity was lower in the GV (p < 0.01), but this value did not differ significantly when compared to the MI stage and MII stage (p > 0.01). The fluorescence intensity in the GVBD stage showed a marked increase and had the greatest difference when compared with other stages (p < 0.01). The fluorescence intensity was strongest during the MI stage (p < 0.01). In oocytes, [Ca2+]i began to increase constantly from the GV stage, reaching the maximum at the MI stage, after which it decreased to a relatively stable level (Fig. 2).
In bovine IVF embryos, Ca2+ was primarily distributed in the cortical ooplasm region before formation of the pronucleus. At 6 h after IVF, Ca2+ was primarily distributed in the cortical ooplasm region with punctate discontinuous distribution and fluorescence intensity in sperm-egg binding sites being higher than in other sites. The circular fluorescent region was the head of the sperm (Fig. 3A). At 10 h after IVF, Ca2+ was continuously distributed in the cortical ooplasm region (Fig. 3B). The distribution of Ca2+ at 14 h was basically the same as at 10 h, but the fluorescence intensity was significantly enhanced and had spread to the cell center (Fig. 3C). At 24 h after IVF, when the female-male pronucleus was formed, Ca2+ was widely distributed in the cells, while Ca2+ fluorescence intensity in the pronucleus was obviously higher than in other regions (Fig. 3D). In 2-cell, 4-cell, 8-cell embryos and the morula, Ca2+ was uniformly distributed throughout the blastomere with high fluorescence intensity (Figs. 3E-H). In blastocysts, the Ca2+ concentration was decreased remarkably and distributed unevenly (Fig. 3I).
At 6 h after IVF, the [Ca2+]i was lowest, after which it increased constantly to a maximum in the 4-cell stage (p < 0.01) and then declined to a stable level. [Ca2+]i did not differ among the IVF 72 h, 120 h and 140 h groups (p > 0.01). Additionally, although the [Ca2+]i differed among the 14 h, 24 h and 160 h groups [Ca2+]i, this difference was not significant (p > 0.01) (Fig. 4).
At 0~24 h after PA, Ca2+ was primarily distributed in the cortical ooplasm region. At 6 h after PA, the Ca2+ fluorescence intensity of the first polar body was higher than that of the cortical ooplasm region (Fig. 5A). At 10 h and 14 h, the Ca2+ distribution was the same as that observed at 6 h, but the fluorescence intensity increased at 10 h (Fig. 5B) and the first polar body was degraded at 14 h (Figs. 5C and D). At 48 h, when cleavage occurred Ca2+ was distributed throughout the blastomere, while the fluorescence intensity was slightly stronger in the nuclear area (Fig. 5E). In 4-cell and 8-cell embryos and morulas, Ca2+ was uniformly distributed throughout the blastomere (Figs. 5F~H), while it was distributed unevenly without obvious regularity in blastocysts (Fig. 5I).
[Ca2+]i was the lowest at 6 h after PA, at which time it differed significantly from the other PA groups (p < 0.01). However, there was no significant difference and change regularity among the other groups (p > 0.01) (Fig. 6).
At 6 h after SCNT, the Ca2+ fluorescence intensity was extremely weak and there was almost no Ca2+ distribution in the reconstructed embryos (Fig. 7A). At 10 h after SCNT, Ca2+ was distributed in the donor nucleus (Fig. 7B). At 14 h, Ca2+ was distributed throughout the cytoplasm of the reconstructed embryos (Fig. 7C). At 24 h Ca2+, was distributed in the donor nucleus and distributed uniformly throughout the cytoplasm of the reconstructed embryos (Fig. 7D). In the 2-cell, 4-cell and 8-cell embryos, Ca2+ was uniformly distributed throughout the blastomere (Figs. 7E~G). In morulas and blastocysts, Ca2+ was distributed unevenly without an obvious pattern (Figs. 7H and I).
[Ca2+]i was lowest at 6 h after SCNT (p < 0.01) and highest at 72 h, while there was no obvious difference between the 24 h and 48 h groups (p > 0.01). The Ca2+ concentration increased with time, reached a peak at 72 h, and subsequently began to decline (Fig. 8).
At 6 h, the Ca2+ fluorescence intensity of SCNT embryos was lowest among IVF and PA embryos (p < 0.01). At 10 h, the Ca2+ fluorescence intensity of the PA embryos was significantly higher than in the IVF and SCNT groups (p < 0.01), while there was no obvious difference among groups at 14 h (p > 0.01). At 24 h, there was a significantly higher fluorescence intensity in the SCNT group than the IVF and PA groups (p < 0.01), and the intensity in the IVF 48 h group was higher than in the other two groups (p < 0.01). At 72 h, PA embryos had the lowest Ca2+ fluorescence intensity among the three groups (p < 0.01). At 120 h and 140 h, the Ca2+ fluorescence intensity of the IVF embryos was the highest (p < 0.01), whereas the three 160 h groups did not differ significantly. Overall, the Ca2+ levels of the IVF and SCNT groups fluctuated significantly, but no obvious changes were observed in the PA group (Fig. 9).
The release of intracellular Ca2+ is a fundamentally important signaling event in oocyte maturation, activation, fertilization and development [31]. Increased [Ca2+]i levels are recognized as the essential factor leading to the release of oocytes from cell cycle arrest [32]. In studies of hamsters [12] and mice [6], a series of spontaneous Ca2+ oscillations occurred in the oocytes after isolation from the ovarian follicles until the GVBD stage.
Some studies have shown that the intracellular [Ca2+]i changed in mouse oocyte meiosis, as did the spatial distribution [28]. Ca2+ was primarily distributed around GV in the GV period, evenly distributed throughout the cell with slightly higher fluorescence intensity during the middle of the GVBD stage, and uniformly distributed in MI stage. Furthermore, during the MII stage, [Ca2+]i decreased in the pronucleus; nevertheless, there was strong distribution and intensity in the polar body [3,21]. In this study, Ca2+ was primarily distributed in the cortical ooplasm in the GV stage. In the GVBD stage, Ca2+ was distributed throughout the cell, but was primarily located in the cortical ooplasm. From the MI to the MII stage, Ca2+ was distributed throughout the oocytes. Ca2+ distribution differed from that of Li et al. [21] in the GVBD stage, and was maintained consistently during other periods. Some studies have shown that [Ca2+]i was related to oocyte cytoplasm maturation during the meiotic process [49]. However, the mechanism of [Ca2+]i in this process is still not clear. The results may be related to animal breeds or experimental time. Further experiments are necessary to determine the specific reasons for these differences.
The calcium-signaling paradigm has been established as follows: a transient increase in cytoplasmic calcium is caused by either calcium influx or internal calcium release and terminated by the resequestration or extrusion of calcium ions by ATP-driven calcium pumps [10]. In most animals, oocytes must undergo a pre-fertilization maturation process [37].
Ca2+-dependent events of egg activation are critical to the initiation of development by eliminating the block to polyspermy, completion of meiosis, onset of interphase prior to mitotic cleavage, and expression of new proteins [24,42]. Studies of PA have shown that the Ca2+ increase is a sufficient trigger for embryonic development [4]. Because extracellular Ca2+ is required for in vitro GVBD and for the first meiotic division, Ca2+ may be transported throughout the plasma membrane, where it plays a functional role in maturation [35].
During either fertilization or artificial activation, intracellular free [Ca2+]i is the initial signal of oocytes activation [48]. To date, fertilized oocytes have been found to undergo a common phenomenon in which cryptozoic [Ca2+]i elevated and spread with in waves from the point of sperm-egg binding to other parts of the egg [43]. Ca2+ oscillation induced by fertilization has been found to be sustained for up to several hours, and does not disappear until pronuclear formation in mice [19]. PA and SCNT embryos were shown to start development in response to certain external stimuli, which simulated the process of fertilization to release Ca2+ and [Ca2+]i oscillations. Full activation of reconstructed embryos is prerequisite to ensure their normal development; therefore, many researchers have investigated the activation mechanism and method of PA and SCNT embryos [38]. This study suggests that the intensity, fluctuations, frequency and duration of Ca2+ during SCNT process closed to the normal fertilization process. To accomplish this, differences in Ca2+ distribution and intensity among reconstructed and IVF embryos were investigated. The results revealed that Ca2+ fluctuation is the initial signal of egg activation, after which a series of reactions occurred including CG release, intracellular pH changes, and supplementation of maternal mRNA. However, the exact causes of these changes requires further study.
It has been suggested that activation is mediated by an increase in Ca2+ that subsequently leads to exit from the second meiotic block [27]. One of the difficulties in attempting to study the effects of Ca2+ transients upon embryo development is the fact that Ca2+ increase is normally associated with and necessary for both fertilization and PA [5]. Following fertilization or activation of oocytes, the intracellular Ca2+ concentration increases rapidly, resulting in cytostatic factor activity disappearing. Next, cyclinB rapidly degrades, inducing the disappearance of maturation promoting factor activity, which results in cells leaving the M phase and subsequently entering the meiotic phase [26].
Ca2+ fluctuation have rule in IVF and SCNT process, but PA. This was likely because PA embryos differ from IVF embryos. The most essential difference between PA and IVF embryos is that PA embryos are produced through activation of female gametes via physical or chemical methods, but IVF embryos are obtained through fertilization of male and female gametes [16]. Many studies have shown that the cytoplasm and nuclear features of PA embryos are defective [30]. The abnormality rate of PA embryonic chromosome haplotypes was shown to be higher than that of IVF embryos and the expression of protein factors are also different [8]. Accordingly, PA embryos have some degree of functional defect, which results in developmental disorders. Calcium waves of different speeds that are generated by different mechanisms are important in embryonic development, in part because Calcium waves have very low diffusion constants inside cells and are strongly bound by cellular calcium buffers [17]. In conclusion, the results of the present study showed that Ca2+ around the nuclear membrane plays important roles in GVBD as well as IVF and SCNT embryonic development.
Figures and Tables
Fig. 1
Ca2+ distribution in bovine oocytes during different phases of in vitro maturation. A: Germinal vesicle (GV) stage; B and C: Germinal vesicle breakdown (GVBD) stage; D: MI stage; E: End of MI stage; F: MII stage.
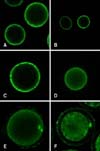
Fig. 2
Comparison of Ca2+ fluorescence intensity in bovine oocytes during different phases of in vitro maturation. p < 0.05 (*) and p < 0.01 (**) indicate significant difference from other groups.

Fig. 3
Ca2+ distribution in bovine in vitro fertilization (IVF) embryos at different times. A: note the round fluorescent zone displayed in the sperm head.

Fig. 4
Comparison of Ca2+ fluorescence intensity in bovine IVF embryos at different times. p < 0.05 (*) and p < 0.01 (**) indicate significant difference from other groups.

Fig. 6
Comparison of Ca2+ fluorescence intensity in bovine parthenogenetic activation embryo at different times. p < 0.05 (*) indicates significant difference from other groups.

Acknowledgments
This research was supported by the 863 National Major Research Program (2006AA10Z198, 2007AA10Z170), National Key Technology R&D Program (2006BAD13B08), National Scientific Foundation of China (30671539) and a project (No. 2008ZX08009-003) from the Ministry of Agriculture of China for transgenic research.
References
1. Bandyopadhyay J, Lee J, Lee J, Lee JI, Yu JR, Jee C, Cho JH, Jung S, Lee MH, Zannoni S, Singson A, Kim DH, Koo HS, Ahnn J. Calcineurin, a Calcium/Calmodulin-dependent protein phosphatase, is involved in movement, fertility, egg laying, and growth in caenorhabditis elegans. Mol Biol Cell. 2002. 13:3281–3293.

2. Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000. 1:11–21.

3. Bi CM, C Y, Dai G, Lu JC, Li CJ, Zhang XR. The Calcium oscillation and distribution before GVBD in mouse oocyte. J Nanjing Norm Univ. 2000. 23:80–83.
4. Boni R, Cuomo A, Tosti E. Developmental potential in bovine oocytes is related to cumulus-oocyte complex grade, calcium current activity, and calcium stores. Biol Reprod. 2002. 66:836–842.

5. Carroll J. The initiation and regulation of Ca2+ signalling at fertilization in mammals. Semin Cell Dev Biol. 2001. 12:37–43.

6. Carroll J, Swann K, Whittingham D, Whitaker M. Spatiotemporal dynamics of intracellular [Ca2+]i oscillations during the growth and meiotic maturation of mouse oocytes. Development. 1994. 120:3507–3517.

7. Carroll J, Swann K. Spontaneous cytosolic calcium oscillations driven by inositol trisphosphate occur during in vitro maturation of mouse oocytes. J Biol Chem. 1992. 267:11196–11201.

8. Choi YH, Love CC, Chung YG, Varner DD, Westhusin ME, Burghardt RC, Hinrichs K. Use of Piezo-driven direct nuclear injection and activation with stallion sperm extract to produce horse nuclear transfer embryos. Theriogenology. 2002. 58:771–774.

9. Ducibella T, Huneau D, Angelichio E, Xu Z, Schultz RM, Kopf GS, Fissore R, Madoux S, Ozil JP. Egg-to-embryo transition is driven by differential responses to Ca2+ oscillation number. Dev Biol. 2002. 250:280–291.

10. Ducibella T, Schultz RM, Ozil JP. Role of calcium signals in early development. Semin Cell Dev Biol. 2006. 17:324–332.

11. Duesbery NS, Masui Y. The role of Ca2+ in progesterone-induced germinal vesicle breakdown of Xenopus laevis oocytes: the synergic effects of microtubule depolymerization and Ca2+. Dev Genes Evol. 1996. 206:110–124.

12. Fujiwara T, Nakada K, Shirakawa H, Miyazaki S. Development of inositol trisphosphate-induced calcium release mechanism during maturation of hamster oocytes. Dev Biol. 1993. 156:69–79.

14. Homa ST, Carroll J, Swann K. The role of calcium in mammalian oocyte maturation and egg activation. Hum Reprod. 1993. 8:1274–1281.
15. Hyslop LA, Nixon VL, Levasseur M, Chapman F, Chiba K, McDougall A, Venables JP, Elliott DJ, Jones KT. Ca2+-promoted cyclin B1 degradation in mouse oocytes requires the establishment of a metaphase arrest. Dev Biol. 2004. 269:206–219.

16. Hyttel P, Viuff D, Laurincik J, Schmidt M, Thomsen PD, Avery B, Callesen H, Rath D, Niemann H, Rosenkranz C, Schellander K, Ochs RL, Greve T. Risks of in-vitro production of cattle and swine embryos: aberrations in chromosome numbers, ribosomal RNA gene activation and perinatal physiology. Hum Reprod. 2000. 15:Suppl 5. 87–97.

17. Ito M, Shikano T, Oda S, Horiguchi T, Tanimoto S, Awaji T, Mitani H, Miyazaki S. Difference in Ca2+ oscillation-inducing activity and nuclear translocation ability of plcz1, an egg-activating sperm factor candidate, between mouse, rat, human, and medaka fish. Biol Reprod. 2008. 78:1081–1090.

19. Kline D, Kline JT. Repetitive calcium transients and the role of calcium in exocytosis and cell cycle activation in the mouse egg. Dev Biol. 1992. 149:80–89.

20. Kong SK, Tsang D, Leung KN, Lee CY. Nuclear envelope acts as a calcium barrier in C6 glioma cells. Biochem Biophys Res Commun. 1996. 218:595–600.

21. Li CJ, Wang B, Fan BQ. The 3-dimensional distribution of free calcium in mouse oocyte during meiotic divisions. Acta Anatomica Sinica. 1995. 26:71–76.
22. Malcuit C, Kurokawa M, Fissore RA. Calcium oscillations and mammalian egg activation. J Cell Physiol. 2006. 206:565–573.

23. Markoulaki S, Kurokawa M, Yoon SY, Matson S, Ducibella T, Fissore R. Comparison of Ca2+ and CaMKII responses in IVF and ICSI in the mouse. Mol Hum Reprod. 2007. 13:265–272.
24. Markoulaki S, Matson S, Abbott AL, Ducibella T. Oscillatory CaMKII activity in mouse egg activation. Dev Biol. 2003. 258:464–474.

25. Mehlmann LM. Oocyte-specific expression of Gpr3 is required for the maintenance of meiotic arrest in mouse oocytes. Dev Biol. 2005. 288:397–404.

26. Mitalipov SM, Zhou Q, Byrne JA, Ji WZ, Norgren RB, Wolf DP. Reprogramming following somatic cell nuclear transfer in primates is dependent upon nuclear remodeling. Hum Reprod. 2007. 22:2232–2242.

27. Miyazaki S, Ito M. Calcium signals for egg activation in mammals. J Pharmacol Sci. 2006. 100:545–552.

28. Moosmang S, Lenhardt P, Haider N, Hofmann F, Wegener JW. Mouse models to study L-type calcium channel function. Pharmacol Ther. 2005. 106:347–355.

29. Nedambale TL, Du F, Xu J, Chaubal SA, Dinnyes A, Groen W, Faber D, Dobrinsky JR, Yang X, Tian XC. Prolonging bovine sperm-oocyte incubation in modified medium 199 improves embryo development rate and the viability of vitrified blastocysts. Theriogenology. 2006. 66:1951–1960.

30. Niemann H, Wrenzycki C. Alterations of expression of developmentally important genes in preimplantation bovine embryos by in vitro culture conditions: implications for subsequent development. Theriogenology. 2000. 53:21–34.

31. Ozil JP, Huneau D. Activation of rabbit oocytes: the impact of the Ca2+ signal regime on development. Development. 2001. 128:917–928.

32. Ozil JP, Markoulaki S, Toth S, Matson S, Banrezes B, Knott JG, Schultz RM, Huneau D, Ducibella T. Egg activation events are regulated by the duration of a sustained [Ca2+]cyt signal in the mouse. Dev Biol. 2005. 282:39–54.

33. Rizos D, Gutiérrez-Adán A, Pérez-Garnelo S, De La Fuente J, Boland MP, Lonergan P. Bovine embryo culture in the presence or absence of serum: implications for blastocyst development, cryotolerance, and messenger RNA expression. Biol Reprod. 2003. 68:236–243.

34. Schultz RM, Kopf GS. 2 Molecular basis of mammalian egg activation. Curr Top Dev Boil. 1995. 30:21–62.

35. Steinhardt R, Zucker R, Schatten G. Intracellular calcium release at fertilization in the sea urchin egg. Dev Biol. 1977. 58:185–196.

36. Steinhardt RA, Epel D. Activation of sea-urchin eggs by a calcium ionophore. Proc Natl Acad Sci USA. 1974. 71:1915–1919.

37. Stricker SA, Smythe TL. Endoplasmic reticulum reorganizations and Ca2+ signaling in maturing and fertilized oocytes of marine protostome worms: the roles of MAPKs and MPF. Development. 2003. 130:2867–2879.

38. Sun L, Hodeify R, Haun S, Charlesworth A, MacNicol AM, Ponnappan S, Ponnappan U, Prigent C, Machaca K. Ca2+ homeostasis regulates Xenopus oocyte maturation. Biol Reprod. 2008. 78:726–735.
39. Sun L, Machaca K. Ca2+cyt negatively regulates the initiation of oocyte maturation. J Cell Biol. 2004. 165:63–75.
40. Tan SJ, Xie ZJ, Shi DS, Xie TS, Li H, Deng YF, Liu JL. Effects of porcine oocyte activation induced by a cytosolic sperm factor. J South China Agric Univ. 2009. 30:78–81.
41. Tombes RM, Simerly C, Borisy GG, Schatten G. Meiosis, egg activation, and nuclear envelope breakdown are differentially reliant on Ca2+, whereas germinal vesicle breakdown is Ca2+ independent in the mouse oocyte. J Cell Biol. 1992. 117:799–811.

42. Tosti E, Boni R, Cuomo A. Ca2+ current activity decreases during meiotic progression in bovine oocytes. Am J Physiol Cell Physiol. 2000. 279:C1795–C1800.
43. Tosti E, Boni R, Cuomo A. Fertilization and activation currents in bovine oocytes. Reproduction. 2002. 124:835–846.

44. Wang CG, Deng MQ, Sun FZ. Intracellular Ca2+ distribution and its role in mouse oocytes maturation. Shi Yan Sheng Wu Xue Bao. 1998. 31:147–153.
45. Whitaker M, Smith J. Introduction. Calcium signals and developmental patterning. Philos Trans R Soc Lond B Biol Sci. 2008. 363:1307–1310.

47. Whitaker M. Calcium signalling in early embryos. Philos Trans R Soc Lond B Biol Sci. 2008. 363:1401–1418.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download