Abstract
The isoflavonol glycoside Talosin A, genistein (GT)-7-α-L-6-deoxy talopyranose (GT-Tal), was first isolated from the culture broth of Kitasatospora kifunensis MJM341. The aim of the present study was to evaluate the oral absorption and metabolism of the newly isolated isoflavonol glycoside, GT-Tal compared to genistin (GT-7-O-β-D-glucopyranoside; GT-Glu). Free GT-Glu and GT-Tal could not be detected prior to enzymatic hydrolysis of the corresponding conjugates in rat plasma. Following oral administration of GT-Tal (15 min), GT-Tal was rapidly absorbed through the gastrointestinal tract and metabolized into GT-Tal conjugates with a mean Cmax of 2.74 µg/mL. GT-Tal was further metabolized to its aglycone, free GT and conjugated GT. After oral administration, GT-Glu was absorbed after being convereted to its aglycone and then further metabolized into its conjugate metabolites (free GT with a mean Cmax of 0.24 mg/mL at 1.25 h; conjugated GT with a mean Cmax of 1.31 mg/mL at 2.00 h). Significant differences in absorption and metabolism of GT-Tal and GT-Glu were observed. GT-Tal was metabolized into its corresponding conjugates or underwent deglycosylation to form GT, whereas GT-Glu was metabolized into its aglycone, GT.
Isoflavones, major dietary components of soybeans, have received much attention because of their health-related and clinical benefits such as estrogenic and anti-oxidative activities as well as promoting natural killer cell activity [1,21,23]. In soybeans and soy-derived foods, genistein (GT; 4',5,7-trihydroxy-isoflavone) is mainly present as a glycoside, genistin (GT-7-O-β-D-glucopyranoside, GT-Glu). It is well-known that colonic bacteria convert GT-Glu into the corresponding aglycone, GT [1,11,21,23].
A large number of novel bioactive metabolites are derived from actinomycetes [10]. The genus Kitasatospora was first identified by Omura et al. [12] as an actinomycete with the unique characteristic of containing meso- and LL-diaminopimelic acid as diamino acid in the cell wall. Several Kitasatospora species have been reported to be producers of novel bioactive compounds with varying structures and beneficial functions including antifungal activity, antitumor activity, immunomodulation, and proteinase inhibition [10,12,18].
Talosin A, GT-7-α-L-6-deoxy talopyranose (GT-Tal), is a new isoflavonol glycoside that was first isolated from the culture broth of Kitasatospora kifunensis MJM341 [7,22]. Soy isoflavones are composed of GT, daidzein, and, to a lesser extent, glycitein. Their biological effects are a topic of considerable current interest due to their health-related and clinical benefits such as estrogenic and anti-oxidative activities [4,6,23]. Unlike other isoflavones, GT-Tal has a strong antifungal activity against Candida albicans, Aspergillus niger, and Cryptococcus neoformans [22]. Moreover, our previous study showed that GT-Tal may exert potent anti-inflammatory effects such as the inhibition of nuclear factor-κB activation, the production of nitric oxide (NO), and the production of proinflammatory cytokines [5]. Understanding the in vivo pharmacokinetics and metabolism of GT-Tal is essential for the biomedical applications of this compound. It is currently unknown whether GT-Tal can be absorbed in its intact form or is hydrolyzed into its aglycone form during the absorption process. Moreover, there are no previous studies that describe a method for analyzing GT-Tal in biological fluids. Therefore, this study evaluated the pharmacokinetics and metabolism of GT-Tal in rats following oral administration of this compound.
GT and GT-Glu were purchased from Sigma (USA) and LC Laboratories (USA), respectively. Formononetin (Sigma, USA) served as an internal standard (IS). Methyl tetra-butyl ether (MTBE), dimethyl sulfoxide (DMSO), acetic acid and ammonium acetate were reagent grade and purchased from Sigma (USA). Hydrolytic enzyme such as α-glucosidase, β-glucosidase, β-glucuronidase/sulfatase from Helix pomatia and sulfatase from Helix pomatia were also purchased from Sigma (USA). HPLC grade methanol and acetonitrile were purchased from Mallinckrodt Baker (USA).
Kitasatospora kifunensis MJM341 was isolated from a soil sample collected in Gyeonggi province, Korea [7]. For the production of GT-Tal, Kitasatospora kifunensis MJM341 was incubated in a 2 L baffled flask containing 500 mL of glucose soluble starch medium for 6 days at 28℃ on a rotary shaker at 200 rpm. For the purification of GT-Tal, the supernatant was extracted from the culture broth with butanol (1:1, v:v) and oily materials were delipidated by partitioning with n-hexane and 15% methanol. The aqueous methanol layer was separated using C18 reversed-phase vacuum flash column chromatography with sequential mixtures of MeOH from 0 to 100%. The fraction eluted with 20% aqueous MeOH was further separated by a C18 reversed-phase column (semi- preparative Symmetry RP-18 column, 10 µm, 7.8 × 300 mm; Waters, USA) to recover the GT-Tal. The mobile phase consisted of a mixture of acetonitril and water (28:72, v:v). The flow rate was set at 4 mL/min with an injection volume of 500 µL.
The experimental protocols were approved by the Institutional Animal Care and Use Committee of Chungnam National University (Korea). Male Sprague-Dawley rats weighing between 160 and 220 g at the age of 6~7 weeks were used for this study. The animals were obtained from Samtako Biokorea (Korea) and acclimated for 1 week before the experiments. The rats were randomly divided into two groups of four male rats each. Oral doses of GT-Glu and GT-Tal were administered by oral gavage in a 50% DMSO solution at 20 mg/kg of bodyweight. Blood samples (0.2 mL each) were collected from the tail vein at the predetermined time points (pre-dosing and 0.25, 0.5, 1, 2, 4, 6, 8, 12, and 24 h after dosing). The collected blood samples were centrifuged at 6,000 rpm for 10 min. Separated plasma was immediately stored at -40℃ until further use.
Various forms of hydrolytic enzymes including α-glucosidase (5 U), β-glucosidase (5 U), β-glucuronidase/sulfatase (10 U) and sulfatase (10 U) were used in a pilot study to determine which enzyme hydrolyzed 6-deoxy-L-talopyranose or D-glucopyranoside. Each enzyme was added to a 500 µL solution containing 5 µg/mL of GT-Tal and GT-Glu at 37℃. The reaction was allowed to proceed for 0.5, 1, 2, 4, 6, and 8 h and stopped by the addition of 200 µL of acetonitrile containing ascorbic acid. Each sample was then analyzed by liquid chromatography/mass spectrometry (LC/MS, Agilent, USA).
For the quantification of total plasma GT or GT-Tal (conjugated + unconjugated forms), 50 µL of plasma samples and 10 µL of IS were incubated overnight at at 37℃ in 0.17 M ammonium acetate (pH 4.6) containing 1,000 U of β-glucuronidase/sulfatase from Helix pomatia (final volume 600 µL) to hydrolyze the conjugates. The reaction was stopped by adding a 4-fold volume of MTBE and then centrifuged at 1,200 ×g for 15 min. The supernatant was transferred into another tube and evaporated at 30℃ under a stream of nitrogen. The residue was reconstituted with a mobile phase. Preliminary studies and the hydrolysis experiments in the present study showed that this protocol results in the complete hydrolyzation of conjugated GT or GT-Tal [9,15,24]. For the quantification of free GT, GT-Glu, or GT-Tal (unconjugated forms) plasma concentration, thawed plasma samples were extracted as described above without prior enzymatic hydrolysis. The amount of conjugated GT or GT-Tal in plasma was calculated as the difference between total GT or GT-Tal and free GT or GT-Tal.
Extracted plasma samples were analyzed on the Agilent 1100 series LC/MS system (USA). Separation was achieved using the Zorbax Extended C18 reverse phase column (3.5 µm, 4.6 mm ×50 mm; Agilent, USA) and the mobile phase consisted of 35% 10 mM ammonium acetate (pH 4.0) and 65% acetonitrile. The flow rate was 0.4 mL/min. The column effluent was introduced into the mass spectrometer using electrospray ionization. Mass spectrometry was performed using the positive ion mode and the selected ion monitoring, and detecting m/z 271 (GT), 433 (GT-Glu), 417 (GT-Tal) and 269 (IS) with z peak width of 0.07 and dwell time of 197 ms. The lower limit of quantitation was 50 ng/mL for GT, GT-Glu, and GT-Tal. The intra- and inter-day variability for the entire standard curve was ~14% for GT, ~14% for GT-Glu, and ~12% for GT-Tal.
GT or GT-Tal plasma concentrations versus time were analyzed by a non-compartmental analysis using WinNonlin 5.2 (Pharsight Corporation, USA). A non-compartmental analysis based on statistical moments was also performed. The area under the plasma concentration-time curve (AUC) and the area under the first moment curve (AUMC) were calculated by the method of trapezoids and extrapolation to infinity. The mean residence time (MRT) was determined by the formula: MRT = AUMC/AUC. Peak plasma concentrations (Cmax) and times to reach peak concentration (tmax) after the oral administration were determined from the individual plasma concentration-time curves.
GT-Glu was quickly hydrolyzed enzymatically to its corresponding aglycon, GT, by α-glucosidase, β-glucosidase, β-glucuronidase/sulfatase, and sulfatase (data not shown). However, GT-Tal was not hydrolyzed to GT by these enzymes.
The plasma concentration-time profiles of total GT-Glu and GT-Tal after oral administration of GT-Glu and GT-Tal are shown in Figs. 1 and 2. The pharmacokinetic parameters for GT-Glu and GT-Tal are summarized in Tables 1 and 2. Free GT-Glu or GT-Tal could not be detected prior to enzymatic hydrolysis of the conjugates after oral administration. GT-Glu was slowly absorbed compared to GT-Tal after being converted into its aglycone and conjugate metabolites (Free-GT, Cmax of 0.24 ± 0.08 µg/mL at 1.25 ± 0.05 h; Conjugated-GT, Cmax of 1.31 ± 0.45 µg/mL at 2.00 ± 0.00 h; Table 1). GT-Tal was rapidly absorbed through the gastrointestinal tract and metabolized into conjugates of GT-Tal with a Cmax of 2.74 ± 0.93 µg/mL at 0.25 ± 0.00 h (Table 2). It was further metabolized into its aglycone, Free-GT, with a Cmax of 0.24 ± 0.01 µg/mL at 1.00 ± 0.00 h and Conjugated-GT with a Cmax of 0.41 ± 0.25 µg/mL at 0.69 ± 0.38 h (Table 2).
The structure of GT-Tal, a newly identified isoflavonol glycoside, is similar to that of GT-Glu [7,22]. Consequently, we observed many differences in the pharmacokinetic profiles of GT-Tal and GT-Glu. GT-Glu was quickly hydrolyzed into its corresponding aglycon, GT, by α-glucosidase, β-glucosidase, β-glucuronidase/sulfatase, and sulfatase. However, GT-Tal was not hydrolyzed to GT by these enzymes. Hydrolytic enzymes may not recognize the alpha linkage between GT and talopyranose because 6-deoxy-L-talose, a stereoisomer at C-4 of L-rhamnose, was characterized as an unusual sugar and has been found a residue of outer membrane lipopolysaccharides in some Gram-negative bacteria such as Escherichia coli, Aeromonas hydrophila, and Actinobacillus actinomycetemcomitans [8,13,19].
GT-Tal was rapidly absorbed through the gastrointestinal tract and rapidly metabolized into its conjugates and aglycone. The rapid absorption of GT-Tal may be due to differences in the specificity of the glycoside. This finding coincides with the report by Hollman et al. showing that the nature of the sugar group affects absorption of quercetin, a major flavonoid [6]. In this study, the majority of absorbed GT-Tal was quickly metabolized into its conjugates while a small amount of absorbed GT-Tal was degraded into its aglycone and conjugates. However, GT-Glu was quickly metabolized to the corresponding less polar aglycone, GT, prior to gastrointestinal absorption and was partly absorbed without previous cleavage.
The difference between GT-Tal and GT-Glu absorption and metabolism rates could be explained by the enzymatic hydrolysis of GT-Glu or GT-Tal. GT-Glu was slowly absorbed and then converted into its aglycone and conjugates. A small amount of GT-Glu was briefly present as an intact molecule in the portal vein circulation while GT derived from GT-Glu would be rapidly absorbed throughout the intestinal tract after oral intake [1,2,17,21]. The slow absorption of GT-Glu compared to that of GT-Tal may be because GT-Glu degradation by gastric hydrochloric acid, β-glycosidase present in the jejunum and ileum, and enterobacteria in the colon requires many steps [1,2,9].
It is noteworthy that the concentration of GT-Tal conjugates was higher than that of its GT conjugates long after oral administration. Despite numerous reports of flavonoid glycoside hydrolysis by intestinal microflora and brush border enzymes [3,11,21] and of glycoside uptake by an intestinal glucose transporters [20], it is still unclear whether or not flavonoid glycosides are absorbed in the intestine of rats in vivo. However, some studies have reported that flavonoids might be absorbed in their glycosylated form via intestinal sugar carriers, whereas the oral absorption of many isoflavonic glycosides was shown to be inhibited due to isoflavone degradation by gastric acid and β-glycosidase present in the jejunum and ileum [6,9,14,16]. It was speculated that intestinal sugar carriers may play a role in GT-Tal absorption and slow conversion into its aglycone.
In conclusion, a number of differences in the absorption and metabolism of GT-Tal and GT-Glu were observed due to their different sugar moiety. GT-Tal was metabolized into its corresponding conjugates or underwent deglycosylation to produce GT. In contrast, GT-Glu was metabolized into its aglycone, GT. The pharmacodynamic effect of the GT-Tal metabolites detected in our study is still unclear. Thus, the in vitro activities of free GT-Tal cannot be directly transferred to in vivo conditions. Further studies are required to demonstrate whether the conjugative metabolites of GT-Tal possess some biological activities that may enable them to serve as an important source of cellular aglycones upon enzymatic hydrolysis at the target site.
Figures and Tables
Fig. 1
Mean plasma concentration-time curves of genistein (GT) after an oral administration of 20 mg/kg GT-7-O-β-D-glucopyranoside (GT-Glu) in rats (n = 4). Filled circles (●): conjugated plasma GT, empty circles (○): free plasma GT. Free GT-Glu was not detected in rat plasma samples after oral administration of genistin. Data are expressed as mean ± SD.

Fig. 2
Mean plasma concentration-time curves of GT-7-α-L-6-deoxy talopyranose (GT-Tal) and GT after an oral administration of 20 mg/kg GT-Tal in rats (n = 4). Filled circles (●): plasma conjugated GT-Tal, empty circles (○): plasma conjugated GT, filled triangles (▼): free plasma GT. Free GT-Tal was not detected in rat plasma samples after oral administration of GT-Tal. Data are expressed as mean ± SD.

Table 1
Pharmacokinetic parameters of GT-Glu in rats after a single oral administration of GT-Glu

*λz: first order rate constant associated with the terminal (log-linear) elimination phase, t1/2λZ: terminal half-life, Cmax: the peak or maximum concentration, Tmax: the time of peak concentration, AUC0-12 h: area under concentration curve (AUC) calculated from time zero to 12 h, AUC0-∞: AUC from time zero extrapolated to infinity, MRT: mean residual time. GT: genistein, GT-Glu: GT-7-O-β-D-glucopyranoside. Data are expressed as mean ± SD.
Table 2
Pharmacokinetic parameters of GT-Tal in rats after a single oral administration of GT-Tal
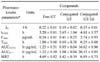
*Parameter definitions are the same as those in Table 1.
Acknowledgments
This work was supported by a grant (20070401-034-023) from the BioGreen 21 Program, Rural Development Administration of Korea. Dr. Jin-Yong Kim was supported by the second stage of the BK21 project.
References
1. Andlauer W, Kolb J, Fürst P. Absorption and metabolism of genistin in the isolated rat small intestine. FEBS Lett. 2000. 475:127–130.

2. Clarke DB, Lloyd AS, Botting NP, Oldfield MF, Needs PW, Wiseman H. Measurement of intact sulfate and glucuronide phytoestrogen conjugates in human urine using isotope dilution liquid chromatography-tandem mass spectrometry with [13C3]isoflavone internal standards. Anal Biochem. 2002. 309:158–172.

3. Day AJ, Cañada FJ, Díaz JC, Kroon PA, Mclauchlan R, Faulds CB, Plumb GW, Morgan MRA, Williamson G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000. 468:166–170.

4. Doerge DR, Chang HC, Churchwell MI, Holder CL. Analysis of soy isoflavone conjugation in vitro and in human blood using liquid chromatography-mass spectrometry. Drug Metab Dispos. 2000. 28:298–307.
5. Hwang YH, Kim MS, Song IB, Lim JH, Park BK, Yun HI. Anti-inflammatory effects of talosin A via inhibition of NF-kappaB activation in lipopolysaccharide-stimulated RAW264.7 cells. Biotechnol Lett. 2009. 31:789–795.

6. Hollman PC, Bijsman MN, van Gameren Y, Cnossen EP, de Vries JH, Katan MB. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radic Res. 1999. 31:569–573.

7. Kim WG, Yoon TM, Kwon HJ, Suh JW. Talosins A and B: new isoflavonol glycosides with potent antifungal activity from Kitasatospora kifunensis MJM341. II. Physicochemical properties and structure determination. J Antibiot (Tokyo). 2006. 59:640–645.

8. Knirel YA, Shashkov AS, Senchenkova SN, Merino S, Tomás JM. Structure of the O-polysaccharide of Aeromonas hydrophila O:34; a case of random O-acetylation of 6-deoxy-L-talose. Carbohydr Res. 2002. 337:1381–1386.

9. Kwon SH, Kang MJ, Huh JS, Ha KW, Lee JR, Lee SK, Lee BS, Han IH, Lee MS, Lee MW, Lee J, Choi YW. Comparison of oral bioavailability of genistein and genistin in rats. Int J Pharm. 2007. 337:148–154.

10. Miyadoh S. Research on antibiotic screening in Japan over the last decade: A producing microorganism approach. Actinomycetologica. 1993. 7:100–106.

11. Nielsen ILF, Williamson G. Review of the factors affecting bioavailability of soy isoflavones in humans. Nutr Cancer. 2007. 57:1–10.

12. Õmura S, Takahashi Y, Iwai Y, Tanaka H. Kitasatosporia, a new genus of the order Actinomycetales. J Antibiot (Tokyo). 1982. 35:1013–1019.
13. Perry MB, MacLean LM, Brisson JR, Wilson ME. Structures of the antigenic O-polysaccharides of lipopolysaccharides produced by Actinobacillus actinomycetemcomitans serotypes a, c, d and e. Eur J Biochem. 1996. 242:682–688.

14. Piskula MK, Yamakoshi J, Iwai Y. Daidzein and genistein but not their glucosides are absorbed from the rat stomach. FEBS Lett. 1999. 447:287–291.

15. Setchell KDR, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, Kirschner AS, Cassidy A, Heubi JE. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001. 131:4 Suppl. 1362S–1375S.

16. Spencer JPE, Chowrimootoo G, Choudhury R, Debnam ES, Srai SK, Rice-Evans C. The small intestine can both absorb and glucuronidate luminal flavonoids. FEBS Lett. 1999. 458:224–230.

17. Steensma A, Faassen-Peters MAW, Noteborn HPJM, Rietjens IMCM. Bioavailability of genistein and its glycoside genistin as measured in the portal vein of freely moving unanesthetized rats. J Agric Food Chem. 2006. 54:8006–8012.

18. Takahshi Y, Õmura S. Isolation of new actinomycete strains for the screening of new bioactive compounds. J Gen Appl Microbiol. 2003. 49:141–154.

19. Torgov VI, Shashkov AS, Kochanowski H, Jann B, Jann K. NMR analysis of the structure of the O88 polysaccharide (O88 antigen) of Escherichia coli O88:K-:H25. Carbohydr Res. 1996. 283:223–227.

20. Walgren RA, Lin JT, Kinne RKH, Walle T. Cellular uptake of dietary flavonoid quercetin 4'-β-glucoside by sodium-dependent glucose transporter SGLT1. J Pharmacol Exp Ther. 2000. 294:837–843.
21. Walsh KR, Haak SJ, Bohn T, Tian Q, Schwartz SJ, Failla ML. Isoflavonoid glucosides are deconjugated and absorbed in the small intestine of human subjects with ileostomies. Am J Clin Nutr. 2007. 85:1050–1056.

22. Yoon TM, Kim JW, Kim JG, Kim WG, Suh JW. Talosins A and B: new isoflavonol glycosides with potent antifungal activity from Kitasatospora kifunensis MJM341. I. Taxonomy, fermentation, isolation, and biological activities. J Antibiot (Tokyo). 2006. 59:633–639.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download